Persisting Cryptococcus yeast species Vishniacozyma victoriae and Cryptococcus neoformans elicit unique airway inflammation in mice following repeated exposure
- PMID: 36864880
- PMCID: PMC9971225
- DOI: 10.3389/fcimb.2023.1067475
Persisting Cryptococcus yeast species Vishniacozyma victoriae and Cryptococcus neoformans elicit unique airway inflammation in mice following repeated exposure
Erratum in
-
Corrigendum: Persisting Cryptococcus yeast species Vishniacozyma victoriae and Cryptococcus neoformans elicit unique airway inflammation in mice following repeated exposure.Front Cell Infect Microbiol. 2024 Feb 16;14:1381148. doi: 10.3389/fcimb.2024.1381148. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38435307 Free PMC article.
Abstract
Background: Allergic airway disease (AAD) is a growing concern in industrialized nations and can be influenced by fungal exposures. Basidiomycota yeast species such as Cryptococcus neoformans are known to exacerbate allergic airway disease; however, recent indoor assessments have identified other Basidiomycota yeasts, including Vishniacozyma victoriae (syn. Cryptococcus victoriae), to be prevalent and potentially associated with asthma. Until now, the murine pulmonary immune response to repeated V. victoriae exposure was previously unexplored.
Objective: This study aimed to compare the immunological impact of repeated pulmonary exposure to Cryptococcus yeasts.
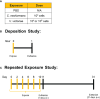
Methods: Mice were repeatedly exposed to an immunogenic dose of C. neoformans or V. victoriae via oropharyngeal aspiration. Bronchoalveolar lavage fluid (BALF) and lungs were collected to examine airway remodeling, inflammation, mucous production, cellular influx, and cytokine responses at 1 day and 21 days post final exposure. The responses to C. neoformans and V. victoriae were analyzed and compared.
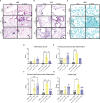
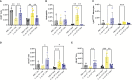
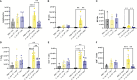
Results: Following repeated exposure, both C. neoformans and V. victoriae cells were still detectable in the lungs 21 days post final exposure. Repeated C. neoformans exposure initiated myeloid and lymphoid cellular infiltration into the lung that worsened over time, as well as an IL-4 and IL-5 response compared to PBS-exposed controls. In contrast, repeated V. victoriae exposure induced a strong CD4+ T cell-driven lymphoid response that started to resolve by 21 days post final exposure.
Discussion: C. neoformans remained in the lungs and exacerbated the pulmonary immune responses as expected following repeated exposure. The persistence of V. victoriae in the lung and strong lymphoid response following repeated exposure were unexpected given its lack of reported involvement in AAD. Given the abundance in indoor environments and industrial utilization of V. victoriae, these results highlight the importance to investigate the impact of frequently detected fungal organisms on the pulmonary response following inhalational exposure. Moreover, it is important to continue to address the knowledge gap involving Basidiomycota yeasts and their impact on AAD.
Keywords: Cryptococcus neoformans; Vishniacozyma victoriae; allergic disease; exposure; fungi; inflammation; yeast.
Copyright © 2023 Rush, Blackwood, Lemons, Green and Croston.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

