Comparative onset of effect of biologics and small molecules in moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis
- PMID: 36864986
- PMCID: PMC9971510
- DOI: 10.1016/j.eclinm.2023.101866
Comparative onset of effect of biologics and small molecules in moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis
Abstract
Background: Onset of effect of advanced therapies is an important parameter due to symptom load and risk of disease complications in moderate-to-severe ulcerative colitis (UC), but comparative data are lacking. Therefore, we aimed to assess the comparative onset of efficacy of biological therapies and small molecules for this patient population.
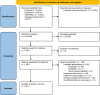
Methods: In this systematic review and network meta-analysis, we searched MEDLINE, Embase, and Cochrane Central Register of Controlled Trials from inception to 24 August 2022, for randomised controlled trials or open-label studies assessing the efficacy of biologics or small molecule drugs within the first six weeks of treatment in adults with UC. The co-primary outcomes were the induction of clinical response and clinical remission at week 2. Network meta-analyses was conducted under the Bayesian framework. This study is registered with PROSPERO: CRD42021250236.
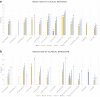
Findings: The systematic literature search identified 20,406 citations, of which 25 studies comprising 11,074 patients fulfilled the eligibility criteria. Upadacitinib ranked highest for induction of clinical response and clinical remission at week 2 and was significantly superior to all agents but tofacitinib, which ranked second highest. Although the rankings remained consistent, no differences between upadacitinib and biological therapies were demonstrated in the sensitivity analyses of partial Mayo clinic score response or resolution of rectal bleeding at week 2. Tumor necrosis factor-α (TNF) inhibitors were significantly superior to vedolizumab and ustekinumab for patient-reported outcome-2 (PRO-2) remission at week 2 in bio-naïve patients. Filgotinib 100 mg, ustekinumab, and ozanimod ranked lowest across all endpoints.
Interpretation: In this network meta-analysis, we found upadacitinib to be significantly superior to all agents but tofacitinib for the induction of clinical response and clinical remission two weeks after treatment initiation. In contrast, ustekinumab and ozanimod ranked lowest. Our findings help to establish the evidence regarding the onset of efficacy of advanced therapies.
Funding: None.
Keywords: Biological therapies; Network meta-analysis; Rapidity; Small molecules; Speed of onset; Ulcerative colitis.
© 2023 The Author(s).
Conflict of interest statement
M.A.: None. E.K.D.: reports research grants from Takeda, paid speaker by MSD, Tillotts. J.B.: reports grants and personal fees from AbbVie, grants and personal fees from Janssen-Cilag, personal fees from Celgene, grants and personal fees from MSD, personal fees from Pfizer, grants and personal fees from Takeda, grants and personal fees from Tillots Pharma, personal fees from Samsung Bioepis, grants and personal fees from Bristol Myers Squibb, grants from Novo Nordisk, personal fees from Pharmacosmos, personal fees from Ferring, personal fees from Galapagos, outside the submitted work. J.G.: None. O.H.N.: None. J.B.S.: has received research grants from Takeda, Janssen, the Danish Research Council, and the Capital Region Denmark, and is national coordinator of studies from AbbVie, Arena Pharmaceuticals, Ely Lilly, and Boehringer Ingelheim. None of these pertain to the research submitted here.
Figures




References
LinkOut - more resources
Full Text Sources

