This is a preprint.
Active zone protein SYD-2/Liprin- α acts downstream of LRK-1/LRRK2 to regulate polarized trafficking of synaptic vesicle precursors through clathrin adaptor protein complexes
- PMID: 36865111
- PMCID: PMC9980171
- DOI: 10.1101/2023.02.26.530068
Active zone protein SYD-2/Liprin- α acts downstream of LRK-1/LRRK2 to regulate polarized trafficking of synaptic vesicle precursors through clathrin adaptor protein complexes
Update in
-
LRK-1/LRRK2 and AP-3 regulate trafficking of synaptic vesicle precursors through active zone protein SYD-2/Liprin-α.PLoS Genet. 2024 May 9;20(5):e1011253. doi: 10.1371/journal.pgen.1011253. eCollection 2024 May. PLoS Genet. 2024. PMID: 38722918 Free PMC article.
Abstract
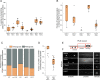
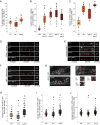
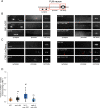
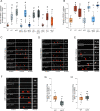
Synaptic vesicle proteins (SVps) are thought to travel in heterogeneous carriers dependent on the motor UNC-104/KIF1A. In C. elegans neurons, we found that some SVps are transported along with lysosomal proteins by the motor UNC-104/KIF1A. LRK-1/LRRK2 and the clathrin adaptor protein complex AP-3 are critical for the separation of lysosomal proteins from SVp transport carriers. In lrk-1 mutants, both SVp carriers and SVp carriers containing lysosomal proteins are independent of UNC-104, suggesting that LRK-1 plays a key role in ensuring UNC-104-dependent transport of SVps. Additionally, LRK-1 likely acts upstream of the AP-3 complex and regulates the membrane localization of AP-3. The action of AP-3 is necessary for the active zone protein SYD-2/Liprin-α to facilitate the transport of SVp carriers. In the absence of the AP-3 complex, SYD-2/Liprin-α acts with UNC-104 to instead facilitate the transport of SVp carriers containing lysosomal proteins. We further show that the mistrafficking of SVps into the dendrite in lrk-1 and apb-3 mutants depends on SYD-2, likely by regulating the recruitment of the AP-1/UNC-101. We propose that SYD-2 acts in concert with both the AP-1 and AP-3 complexes to ensure polarized trafficking of SVps.
Figures


References
-
- Aravamudan B., Fergestad T., Davis W. S., Rodesch C. K. and Broadie K. (1999) ‘Drosophila UNC-13 is essential for synaptic transmission’, Nat Neurosci, 2(11), pp. 965–71. - PubMed
-
- Brockmann M. M., Zarebidaki F., Camacho M., Grauel M. K., Trimbuch T., Südhof T. C. and Rosenmund C. (2020) ‘A Trio of Active Zone Proteins Comprised of RIM-BPs, RIMs, and Munc13s Governs Neurotransmitter Release’, Cell Rep, 32(5), pp. 107960. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
