This is a preprint.
New tuberculosis vaccines in India: Modelling the potential health and economic impacts of adolescent/adult vaccination with M72/AS01 E and BCG-revaccination
- PMID: 36865172
- PMCID: PMC9980245
- DOI: 10.1101/2023.02.24.23286406
New tuberculosis vaccines in India: Modelling the potential health and economic impacts of adolescent/adult vaccination with M72/AS01 E and BCG-revaccination
Update in
-
New tuberculosis vaccines in India: modelling the potential health and economic impacts of adolescent/adult vaccination with M72/AS01E and BCG-revaccination.BMC Med. 2023 Aug 4;21(1):288. doi: 10.1186/s12916-023-02992-7. BMC Med. 2023. PMID: 37542319 Free PMC article.
Abstract
Background India had an estimated 2.9 million tuberculosis cases and 506 thousand deaths in 2021. Novel vaccines effective in adolescents and adults could reduce this burden. M72/AS01E and BCG-revaccination have recently completed Phase IIb trials and estimates of their population-level impact are needed. We estimated the potential health and economic impact of M72/AS01E and BCG-revaccination in India and investigated the impact of variation in vaccine characteristics and delivery strategies. Methods We developed an age-stratified compartmental tuberculosis transmission model for India calibrated to country-specific epidemiology. We projected baseline epidemiology to 2050 assuming no-new-vaccine introduction, and M72/AS01E and BCG-revaccination scenarios over 2025-2050 exploring uncertainty in product characteristics (vaccine efficacy, mechanism of effect, infection status required for vaccine efficacy, duration of protection) and implementation (achieved vaccine coverage and ages targeted). We estimated reductions in tuberculosis cases and deaths by each scenario compared to no-new-vaccine introduction, as well as costs and cost-effectiveness from health-system and societal perspectives. Results M72/AS01E scenarios were predicted to avert 40% more tuberculosis cases and deaths by 2050 compared to BCG-revaccination scenarios. Cost-effectiveness ratios for M72/AS01E vaccines were around seven times higher than BCG-revaccination, but nearly all scenarios were cost-effective. The estimated average incremental cost was US$190 million for M72/AS01E and US$23 million for BCG-revaccination per year. Sources of uncertainty included whether M72/AS01E was efficacious in uninfected individuals at vaccination, and if BCG-revaccination could prevent disease. Conclusions M72/AS01E and BCG-revaccination could be impactful and cost-effective in India. However, there is great uncertainty in impact, especially given unknowns surrounding mechanism of effect and infection status required for vaccine efficacy. Greater investment in vaccine development and delivery is needed to resolve these unknowns in vaccine product characteristics.
Conflict of interest statement
Competing interests
RCH reports employment by Sanofi Pasteur, unrelated to tuberculosis and outside the submitted work. NAM received consulting fees from The Global Fund to Fight AIDS, Tuberculosis and Malaria, and the WHO, and reports funding to their institution from the U.S. Centers for Disease Control and Prevention, the Bill & Melinda Gates Foundation, NIH, and U.S. Council of State and Territorial Epidemiologists. RGW is also funded for other work by the Wellcome Trust (218261/Z/19/Z), NIH (1R01AI147321–01), EDCTP (RIA208D-2505B), UK MRC (CCF 17–7779 via SET Bloomsbury), ESRC (ES/P008011/1), BMGF (OPP1084276, OPP1135288 & INV-001754), and the WHO. All other authors declare no conflicts of interest.
Figures
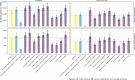


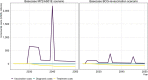
Similar articles
-
New tuberculosis vaccines in India: modelling the potential health and economic impacts of adolescent/adult vaccination with M72/AS01E and BCG-revaccination.BMC Med. 2023 Aug 4;21(1):288. doi: 10.1186/s12916-023-02992-7. BMC Med. 2023. PMID: 37542319 Free PMC article.
-
The potential health and economic impacts of new tuberculosis vaccines under varying delivery strategies in Delhi and Gujarat, India: a modelling study.medRxiv [Preprint]. 2023 Sep 27:2023.09.27.23296211. doi: 10.1101/2023.09.27.23296211. medRxiv. 2023. Update in: Lancet Reg Health Southeast Asia. 2024 May 16;31:100424. doi: 10.1016/j.lansea.2024.100424. PMID: 37808744 Free PMC article. Updated. Preprint.
-
Modelling the health and economic impacts ofM72/AS01E vaccination and BCG-revaccination: Estimates for South Africa.Vaccine. 2024 Feb 27;42(6):1311-1318. doi: 10.1016/j.vaccine.2024.01.072. Epub 2024 Feb 2. Vaccine. 2024. PMID: 38307747
-
The Systematic Review and Meta-Analysis on the Immunogenicity and Safety of the Tuberculosis Subunit Vaccines M72/AS01E and MVA85A.Front Immunol. 2020 Oct 8;11:1806. doi: 10.3389/fimmu.2020.01806. eCollection 2020. Front Immunol. 2020. PMID: 33133057 Free PMC article.
-
New tuberculosis vaccines: advances in clinical development and modelling.J Intern Med. 2020 Dec;288(6):661-681. doi: 10.1111/joim.13197. J Intern Med. 2020. PMID: 33128834 Review.
References
-
- World Health Organization. Global Tuberculosis Report 2022. 2022.
-
- World Health Organization. Tuberculosis data. 2022. https://www.who.int/teams/global-tuberculosis-programme/data. Accessed 13 Dec 2022.
-
- National Tuberculosis Elimination Programme. National Strategic Plan to End Tuberculosis in India 2020–2025. New Delhi: Ministry of Health with Family Welfare, 2020.
-
- Ministry of Health and Family Welfare, Government of India. India TB Report 2022. 2022.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
