This is a preprint.
Feasibility and preliminary efficacy of a virtual reality intervention targeting distress and anxiety in primary brain tumor patients at the time of clinical evaluation: Study protocol for a phase 2 clinical trial
- PMID: 36865245
- PMCID: PMC9980195
- DOI: 10.21203/rs.3.rs-2521990/v1
Feasibility and preliminary efficacy of a virtual reality intervention targeting distress and anxiety in primary brain tumor patients at the time of clinical evaluation: Study protocol for a phase 2 clinical trial
Update in
-
Feasibility and preliminary efficacy of a virtual reality intervention targeting distress and anxiety in primary brain tumor patients at the time of clinical evaluation: Study protocol for a phase 2 clinical trial.BMC Cancer. 2023 Mar 21;23(1):262. doi: 10.1186/s12885-023-10671-2. BMC Cancer. 2023. PMID: 36944930 Free PMC article.
Abstract
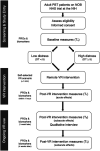
Background: Primary brain tumor (PBT) patients experience higher levels of distress and anxiety than other solid tumor patients, particularly at the time of clinical evaluation when uncertainty about disease status is high ("scanxiety"). There is promising evidence supporting use of virtual reality (VR) to target psychological symptoms in other solid tumor patients, though PBT patients have not been studied extensively in this context. The primary aim of this phase 2 clinical trial is to establish the feasibility of a remote VR-based relaxation intervention for a PBT population, with secondary aims designed to determine preliminary efficacy of improving distress and anxiety symptoms. Methods: PBT patients (N=120) with upcoming MRI scans and clinical appointments who meet eligibility will be recruited to participate in a single arm trial conducted remotely through the NIH. Following completion of baseline assessments, participants will complete a 5-minute VR intervention via telehealth using a head-mounted immersive device while under supervision of the research team. Following the intervention, over the course of 1 month patients can use VR at their discretion with follow-up assessments done immediately post-VR intervention, as well as 1 week and 4 weeks later. Additionally, a qualitative phone interview will be conducted to assess patient satisfaction with the intervention. Discussion: Use of immersive VR is an innovative interventional approach to target distress and scanxiety symptoms in PBT patients who are at high risk for experiencing these symptoms leading into their clinical appointments. Findings from this study may inform design of a future multicenter randomized VR trial for PBT patients and may aid in development of similar interventions for other oncology populations. Trial Registration: clinicaltrials.gov (NCT04301089), registered 9 March 2020.
Conflict of interest statement
Figures
References
-
- National Comprehensive Cancer Network (NCCN). Distress management. Practice Guidelines in Oncology. Fort Washington, PA: NCCN, 2002.
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical

