IgA Nephropathy: Pleiotropic impact of Epstein-Barr virus infection on immunopathogenesis and racial incidence of the disease
- PMID: 36865536
- PMCID: PMC9973316
- DOI: 10.3389/fimmu.2023.1085922
IgA Nephropathy: Pleiotropic impact of Epstein-Barr virus infection on immunopathogenesis and racial incidence of the disease
Abstract
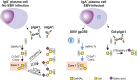
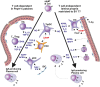
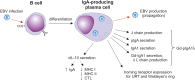
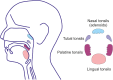
IgA nephropathy (IgAN) is an autoimmune disease in which poorly galactosylated IgA1 is the antigen recognized by naturally occurring anti-glycan antibodies, leading to formation of nephritogenic circulating immune complexes. Incidence of IgAN displays geographical and racial disparity: common in Europe, North America, Australia, and east Asia, uncommon in African Americans, many Asian and South American countries, Australian Aborigines, and rare in central Africa. In analyses of sera and cells from White IgAN patients, healthy controls, and African Americans, IgAN patients exhibited substantial enrichment for IgA-expressing B cells infected with Epstein-Barr virus (EBV), leading to enhanced production of poorly galactosylated IgA1. Disparities in incidence of IgAN may reflect a previously disregarded difference in the maturation of the IgA system as related to the timing of EBV infection. Compared with populations with higher incidences of IgAN, African Americans, African Blacks, and Australian Aborigines are more frequently infected with EBV during the first 1-2 years of life at the time of naturally occurring IgA deficiency when IgA cells are less numerous than in late childhood or adolescence. Therefore, in very young children EBV enters "non-IgA" cells. Ensuing immune responses prevent infection of IgA B cells during later exposure to EBV at older ages. Our data implicate EBV-infected cells as the source of poorly galactosylated IgA1 in circulating immune complexes and glomerular deposits in patients with IgAN. Thus, temporal differences in EBV primo-infection as related to naturally delayed maturation of the IgA system may contribute to geographic and racial variations in incidence of IgAN.
Keywords: Epstein-Barr virus; IgA nephropathy; IgA system maturation; age of infection; galactose-deficient IgA1; virus spread.
Copyright © 2023 Mestecky, Julian and Raska.
Conflict of interest statement
BAJ is a co-founder and co-owner of and consultant for Reliant Glycosciences, LLC and a co-inventor on US patent application 14/318,082 assigned to UAB Research Foundation that distributes royalties to the inventors. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Role of Epstein-Barr Virus in Pathogenesis and Racial Distribution of IgA Nephropathy.Front Immunol. 2020 Feb 28;11:267. doi: 10.3389/fimmu.2020.00267. eCollection 2020. Front Immunol. 2020. PMID: 32184780 Free PMC article.
-
IgA nephropathy: characterization of IgG antibodies specific for galactose-deficient IgA1.Contrib Nephrol. 2007;157:129-33. doi: 10.1159/000102454. Contrib Nephrol. 2007. PMID: 17495450 Review.
-
Galactose-deficient IgA1 in African Americans with IgA nephropathy: serum levels and heritability.Clin J Am Soc Nephrol. 2010 Nov;5(11):2069-74. doi: 10.2215/CJN.03270410. Epub 2010 Jul 15. Clin J Am Soc Nephrol. 2010. PMID: 20634323 Free PMC article.
-
Immunostaining of galactose-deficient IgA1 by KM55 is not specific for immunoglobulin A nephropathy.Clin Immunol. 2020 Aug;217:108483. doi: 10.1016/j.clim.2020.108483. Epub 2020 May 30. Clin Immunol. 2020. PMID: 32479989
-
IgA nephropathy enigma.Clin Immunol. 2016 Nov;172:72-77. doi: 10.1016/j.clim.2016.07.011. Epub 2016 Jul 18. Clin Immunol. 2016. PMID: 27444044 Free PMC article. Review.
Cited by
-
The Role of Epstein-Barr Virus in the Pathogenesis of Autoimmune Diseases.Medicina (Kaunas). 2025 Jun 25;61(7):1148. doi: 10.3390/medicina61071148. Medicina (Kaunas). 2025. PMID: 40731778 Free PMC article. Review.
-
The evolving understanding of systemic mechanisms in organ-specific IgA nephropathy: a focus on gut-kidney crosstalk.Theranostics. 2025 Jan 1;15(2):656-681. doi: 10.7150/thno.104631. eCollection 2025. Theranostics. 2025. PMID: 39744688 Free PMC article. Review.
-
Exploring the causal role of pathogen-derived antibodies in major urinary and kidney diseases: Insights from generalized summary data-based Mendelian randomization.Virulence. 2025 Dec;16(1):2473631. doi: 10.1080/21505594.2025.2473631. Epub 2025 Mar 11. Virulence. 2025. PMID: 40033947 Free PMC article.
-
Identification of key necroptosis-related genes and immune landscape in patients with immunoglobulin A nephropathy.BMC Nephrol. 2024 Dec 18;25(1):459. doi: 10.1186/s12882-024-03885-4. BMC Nephrol. 2024. PMID: 39696012 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

