Validation of thermal dynamics during Hyperthermic IntraPEritoneal Chemotherapy simulations using a 3D-printed phantom
- PMID: 36865797
- PMCID: PMC9971922
- DOI: 10.3389/fonc.2023.1102242
Validation of thermal dynamics during Hyperthermic IntraPEritoneal Chemotherapy simulations using a 3D-printed phantom
Abstract
Introduction: CytoReductive Surgery (CRS) followed by Hyperthermic IntraPeritoneal Chemotherapy (HIPEC) is an often used strategy in treating patients diagnosed with peritoneal metastasis (PM) originating from various origins such as gastric, colorectal and ovarian. During HIPEC treatments, a heated chemotherapeutic solution is circulated through the abdomen using several inflow and outflow catheters. Due to the complex geometry and large peritoneal volume, thermal heterogeneities can occur resulting in an unequal treatment of the peritoneal surface. This can increase the risk of recurrent disease after treatment. The OpenFoam-based treatment planning software that we developed can help understand and map these heterogeneities.
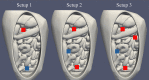
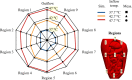
Methods: In this study, we validated the thermal module of the treatment planning software with an anatomically correct 3D-printed phantom of a female peritoneum. This phantom is used in an experimental HIPEC setup in which we varied catheter positions, flow rate and inflow temperatures. In total, we considered 7 different cases. We measured the thermal distribution in 9 different regions with a total of 63 measurement points. The duration of the experiment was 30 minutes, with measurement intervals of 5 seconds.
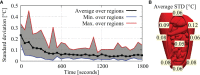
Results: Experimental data were compared to simulated thermal distributions to determine the accuracy of the software. The thermal distribution per region compared well with the simulated temperature ranges. For all cases, the absolute error was well below 0.5°C near steady-state situations and around 0.5°C, for the entire duration of the experiment.
Discussion: Considering clinical data, an accuracy below 0.5°C is adequate to provide estimates of variations in local treatment temperatures and to help optimize HIPEC treatments.
Keywords: cancer biology; computational fluid dynamics (CFD); computational modeling; hyperthermic intrapertioneal chemotherapy (HIPEC); translational research; treatment planning software; validation.
Copyright © 2023 Löke, Kok, Helderman, Franken, Oei, Tuynman, Zweije, Sijbrands, Tanis and Crezee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Demonstration of treatment planning software for hyperthermic intraperitoneal chemotherapy in a rat model.Int J Hyperthermia. 2021;38(1):38-54. doi: 10.1080/02656736.2020.1852324. Int J Hyperthermia. 2021. PMID: 33487083
-
Application of HIPEC simulations for optimizing treatment delivery strategies.Int J Hyperthermia. 2023;40(1):2218627. doi: 10.1080/02656736.2023.2218627. Int J Hyperthermia. 2023. PMID: 37455017
-
A Four-Inflow Construction to Ensure Thermal Stability and Uniformity during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Rats.Cancers (Basel). 2020 Nov 26;12(12):3516. doi: 10.3390/cancers12123516. Cancers (Basel). 2020. PMID: 33255921 Free PMC article.
-
Effect of hyperthermic intraperitoneal chemotherapy in combination with cytoreductive surgery on the prognosis of patients with colorectal cancer peritoneal metastasis: a systematic review and meta-analysis.World J Surg Oncol. 2022 Jun 14;20(1):200. doi: 10.1186/s12957-022-02666-3. World J Surg Oncol. 2022. PMID: 35701802 Free PMC article.
-
Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: a systematic review.Eur J Cancer. 2020 Mar;127:76-95. doi: 10.1016/j.ejca.2019.10.034. Epub 2020 Jan 24. Eur J Cancer. 2020. PMID: 31986452
Cited by
-
Computational Evaluation of Improved HIPEC Drug Delivery Kinetics via Bevacizumab-Induced Vascular Normalization.Pharmaceutics. 2025 Jan 23;17(2):155. doi: 10.3390/pharmaceutics17020155. Pharmaceutics. 2025. PMID: 40006522 Free PMC article.
-
Transforming hyperthermic intraperitoneal chemotherapy: using computer simulation to improve HIPEC treatments.J Gastrointest Oncol. 2024 Dec 31;15(6):2745-2747. doi: 10.21037/jgo-24-755. Epub 2024 Dec 18. J Gastrointest Oncol. 2024. PMID: 39816021 Free PMC article. No abstract available.
References
-
- Privalov A, Vazenin A, Chernova L, Taratonov A, Gubaydulina T. Ovarian cancer with peritoneal carcinomatosis: Comparing of photodynamic treatment and hipec. J Clin Oncol (2017) 35:e17040–0. doi: 10.1200/JCO.2017.35.15_suppl.e17040 - DOI
-
- Verwaal VJ, van Ruth S, de Bree E, van Slooten GW, van Tinteren H, Boot H, et al. . Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol (2003) 21:3737–43. doi: 10.1200/JCO.2003.04.187 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

