Tethered platelet capture provides a mechanism for restricting circulating platelet activation to the wound site
- PMID: 36865905
- PMCID: PMC9971284
- DOI: 10.1016/j.rpth.2023.100058
Tethered platelet capture provides a mechanism for restricting circulating platelet activation to the wound site
Abstract
Background: Puncture wounding is a longstanding challenge to human health for which understanding is limited, in part, by a lack of detailed morphological data on how the circulating platelet capture to the vessel matrix leads to sustained, self-limiting platelet accumulation.
Objectives: The objective of this study was to produce a paradigm for self-limiting thrombus growth in a mouse jugular vein model.
Methods: Data mining of advanced electron microscopy images was performed from authors' laboratories.
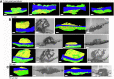
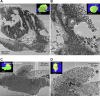
Results: Wide-area transmission electron mcrographs revealed initial platelet capture to the exposed adventitia resulted in localized patches of degranulated, procoagulant-like platelets. Platelet activation to a procoagulant state was sensitive to dabigatran, a direct-acting PAR receptor inhibitor, but not to cangrelor, a P2Y12 receptor inhibitor. Subsequent thrombus growth was sensitive to both cangrelor and dabigatran and sustained by the capture of discoid platelet strings first to collagen-anchored platelets and later to loosely adherent peripheral platelets. Spatial examination indicated that staged platelet activation resulted in a discoid platelet tethering zone that was pushed progressively outward as platelets converted from one activation state to another. As thrombus growth slowed, discoid platelet recruitment became rare and loosely adherent intravascular platelets failed to convert to tightly adherent platelets.
Conclusions: In summary, the data support a model that we term Capture and Activate, in which the initial high platelet activation is directly linked to the exposed adventitia, all subsequent tethering of discoid platelets is to loosely adherent platelets that convert to tightly adherent platelets, and self-limiting, intravascular platelet activation over time is the result of decreased signaling intensity.
Keywords: image analysis; platelets; puncture wound hemostasis; serial block face electron microscopy; thrombus formation; wide-area transmission electron microscopy.
© 2023 The Authors.
Figures






Similar articles
-
Role of collagen-adherent platelets in mediating fibrin formation in flowing whole blood.Blood. 1995 Nov 15;86(10):3815-22. Blood. 1995. PMID: 7579349
-
Intact platelet membranes, not platelet-released microvesicles, support the procoagulant activity of adherent platelets.Arterioscler Thromb. 1993 Nov;13(11):1613-22. doi: 10.1161/01.atv.13.11.1613. Arterioscler Thromb. 1993. PMID: 8218102
-
Venous puncture wound hemostasis results in a vaulted thrombus structured by locally nucleated platelet aggregates.Commun Biol. 2021 Sep 16;4(1):1090. doi: 10.1038/s42003-021-02615-y. Commun Biol. 2021. PMID: 34531522 Free PMC article.
-
Platelet receptor signaling in thrombus formation.J Mol Med (Berl). 2011 Feb;89(2):109-21. doi: 10.1007/s00109-010-0691-5. Epub 2010 Nov 7. J Mol Med (Berl). 2011. PMID: 21058007 Review.
-
Platelet Signaling in Primary Haemostasis and Arterial Thrombus Formation: Part 1.Hamostaseologie. 2018 Nov;38(4):203-210. doi: 10.1055/s-0038-1675144. Epub 2018 Oct 23. Hamostaseologie. 2018. PMID: 30352470 Review.
Cited by
-
Puncture Wound Hemostasis and Preparation of Samples for Montaged Wide-Area Electron Microscopy Analysis.J Vis Exp. 2024 May 24;(207):10.3791/66479. doi: 10.3791/66479. J Vis Exp. 2024. PMID: 38856226 Free PMC article.
-
Contrasting Effects of Platelet GPVI Deletion Versus Syk Inhibition on Mouse Jugular Vein Puncture Wound Structure.Int J Mol Sci. 2025 May 1;26(9):4294. doi: 10.3390/ijms26094294. Int J Mol Sci. 2025. PMID: 40362537 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

