Adverse events of tumor necrosis factor alpha inhibitors for the treatment of ankylosing spondylitis: A meta-analysis of randomized, placebo-controlled trials
- PMID: 36865909
- PMCID: PMC9972296
- DOI: 10.3389/fphar.2023.1084614
Adverse events of tumor necrosis factor alpha inhibitors for the treatment of ankylosing spondylitis: A meta-analysis of randomized, placebo-controlled trials
Abstract
Objective: Tumor necrosis factor alpha inhibitors (TNFi) have shown substantial efficacy in alleviating and treating ankylosing spondylitis (AS). However, the heightened interest is accompanied by concerns over adverse events. In this meta-analysis, we analyzed both serious and common adverse events in patients treated with tumor necrosis factor alpha inhibitors compared with those in the placebo group. Methods: We searched for clinical trials in PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, Wanfang Data, and VIP Data. Studies were selected based on strict inclusion and exclusion criteria. Only randomized, placebo-controlled trials were included in the final analysis. RevMan 5.4 software was used for performing meta-analyses. Results: A total of 18 randomized controlled trials recruiting 3,564 patients with ankylosing spondylitis were included, with overall moderate to high methodological quality. Compared with the placebo group, the incidences showed no difference and were only slightly increased numerically for serious adverse events, serious infections, upper respiratory tract infection, and malignancies in patients treated with tumor necrosis factor alpha inhibitors. However, tumor necrosis factor alpha inhibitor treatment significantly increased the incidence of overall adverse events, nasopharyngitis, headache, and injection-site reactions in ankylosing spondylitis patients when compared with placebo. Conclusion: The available data indicated that ankylosing spondylitis patients who received tumor necrosis factor alpha inhibitors had no significantly increased risks of serious adverse events when compared with the placebo group. However, tumor necrosis factor alpha inhibitors significantly increased the incidence rate of common adverse events, including nasopharyngitis, headache, and injection-site reactions. Large-scale and long-term follow-up clinical trials are still necessary to further investigate the safety of tumor necrosis factor alpha inhibitors in ankylosing spondylitis treatment.
Keywords: adverse events; ankylosing spondylitis; infection; randomized controlled trial; tumor necrosis factor alpha inhibitors.
Copyright © 2023 Feng, Zhao, Kuang, Dai, Cen and Qin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
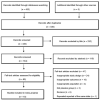




References
-
- Bao C., Huang F., Khan M. A., Fei K., Wu Z., Han C., et al. (2014). Safety and efficacy of golimumab in Chinese patients with active ankylosing spondylitis: 1-year results of a multicentre, randomized, double-blind, placebo-controlled phase III trial. Rheumatol. Oxf. 53, 1654–1663. 10.1093/rheumatology/keu132 - DOI - PubMed
-
- Bongartz T., Sutton A. J., Sweeting M. J., Buchan I., Matteson E. L., Montori V. (2006). Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295, 2275–2285. 10.1001/jama.295.19.2275 - DOI - PubMed
-
- Burmester G. R., Panaccione R., Gordon K. B., McIlraith M. J., Lacerda A. P. (2013). Adalimumab: Long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann. Rheum. Dis. 72, 517–524. 10.1136/annrheumdis-2011-201244 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

