A facile cost-effective electrolyte-assisted approach and comparative study towards the Greener synthesis of silica nanoparticles
- PMID: 36866261
- PMCID: PMC9972527
- DOI: 10.1039/d2na00872f
A facile cost-effective electrolyte-assisted approach and comparative study towards the Greener synthesis of silica nanoparticles
Abstract
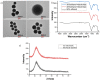
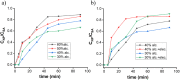
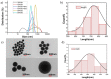
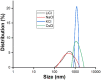
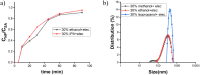
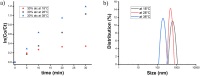
Nowadays, silica nanoparticles are gaining tremendous importance because of their wide applications across different domains such as drug delivery, chromatography, biosensors, and chemosensors. The synthesis of silica nanoparticles generally requires a high percentage composition of organic solvent in an alkali medium. The eco-friendly synthesis of silica nanoparticles in bulk amounts can help save the environment and is cost-effective. Herein, efforts have been made to minimize the concentration of organic solvents used during synthesis via the addition of a low concentration of electrolytes, e.g., NaCl. The effects of electrolytes and solvent concentrations on nucleation kinetics, particle growth, and particle size were investigated. Ethanol was used as a solvent in various concentrations, ranging from 60% to 30%, and to optimize and validate the reaction conditions, isopropanol and methanol were also utilized as solvents. The concentration of aqua-soluble silica was determined using the molybdate assay to establish reaction kinetics, and this approach was also utilized to quantify the relative concentration changes in particles throughout the synthesis. The prime feature of the synthesis is the reduction in organic solvent usage by up to 50% using 68 mM NaCl. The surface zeta potential was reduced after the addition of an electrolyte, which made the condensation process faster and helped reaching the critical aggregation concentration in a shorter time. The effect of temperature was also monitored, and we obtained homogeneous and uniform nanoparticles by increasing the temperature. We found that it is possible to tune the size of the nanoparticles by changing the concentration of electrolytes and the temperature of the reaction using an eco-friendly approach. The overall cost of the synthesis can also be reduced by ∼35% by adding electrolytes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors claim no conflict of interest for this work.
Figures

References
-
- Slowing I. I. Trewyn B. G. Giri S. Lin V. Y. Mesoporous Silica Nanoparticles for Drug Delivery and Biosensing Applications. Adv. Funct. Mater. 2007;17:1225–1236. doi: 10.1002/adfm.200601191. - DOI
-
- Mathelié-Guinlet M. Cohen-Bouhacina T. Gammoudi I. Martin A. Beven L. Delville M. H. Grauby-Heywang C. Silica nanoparticles-assisted electrochemical biosensor for the rapid, sensitive and specific detection of Escherichia coli. Sens. Actuators, B. 2019;292:314–320. doi: 10.1016/j.snb.2019.03.144. - DOI
LinkOut - more resources
Full Text Sources

