Temperature increase modifies susceptibility to Verticillium wilt in Medicago spp and may contribute to the emergence of more aggressive pathogenic strains
- PMID: 36866360
- PMCID: PMC9972977
- DOI: 10.3389/fpls.2023.1109154
Temperature increase modifies susceptibility to Verticillium wilt in Medicago spp and may contribute to the emergence of more aggressive pathogenic strains
Abstract
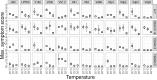
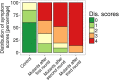
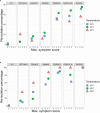
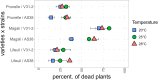
Global warming is expected to have a direct impact on plant disease patterns in agro-eco-systems. However, few analyses report the effect of moderate temperature increase on disease severity due to soil-borne pathogens. For legumes, modifications of root plant-microbe interactions either mutualistic or pathogenic due to climate change may have dramatic effects. We investigated the effect of increasing temperature on the quantitative disease resistance to Verticillium spp., a major soil-borne fungal pathogen, in the model legume Medicago truncatula and the crop M. sativa. First, twelve pathogenic strains isolated from various geographical origin were characterized with regard to their in vitro growth and pathogenicity at 20°C, 25°C and 28°C. Most of them exhibited 25°C as the optimum temperature for in vitro parameters, and between 20°C and 25°C for pathogenicity. Second, a V. alfalfae strain was adapted to the higher temperature by experimental evolution, i.e. three rounds of UV mutagenesis and selection for pathogenicity at 28°C on a susceptible M. truncatula genotype. Inoculation of monospore isolates of these mutants on resistant and susceptible M. truncatula accessions revealed that at 28°C they were all more aggressive than the wild type strain, and that some had acquired the ability to cause disease on resistant genotype. Third, one mutant strain was selected for further studies of the effect of temperature increase on the response of M. truncatula and M. sativa (cultivated alfalfa). The response of seven contrasted M. truncatula genotypes and three alfalfa varieties to root inoculation was followed using disease severity and plant colonization, at 20°C, 25°C and 28°C. With increasing temperature, some lines switched from resistant (no symptoms, no fungus in the tissues) to tolerant (no symptoms but fungal growth into the tissues) phenotypes, or from partially resistant to susceptible. Further studies in greenhouse evidence the reduction in plant fitness due to disease in susceptible lines. We thus report that root pathogenic interactions are affected by anticipated global warming, with trends towards increased plant susceptibility and larger virulence for hot-adapted strains. New threats due to hot-adapted strains of soil-borne pathogens, with possibly wider host range and increased aggressiveness, might occur.
Keywords: aggressiveness; experimental evolution; global warming; mutation; plant disease; temperature-adapted pathogens; virulence.
Copyright © 2023 Sbeiti, Mazurier, Ben, Rickauer and Gentzbittel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Medicago truncatula quantitative resistance to a new strain of Verticillium alfalfae from Iran revealed by a genome-wide association study.Front Plant Sci. 2023 Apr 14;14:1125551. doi: 10.3389/fpls.2023.1125551. eCollection 2023. Front Plant Sci. 2023. PMID: 37123855 Free PMC article.
-
Natural diversity in the model legume Medicago truncatula allows identifying distinct genetic mechanisms conferring partial resistance to Verticillium wilt.J Exp Bot. 2013 Jan;64(1):317-32. doi: 10.1093/jxb/ers337. Epub 2012 Dec 3. J Exp Bot. 2013. PMID: 23213135 Free PMC article.
-
Quantitative Resistance to Verticillium Wilt in Medicago truncatula Involves Eradication of the Fungus from Roots and Is Associated with Transcriptional Responses Related to Innate Immunity.Front Plant Sci. 2016 Sep 29;7:1431. doi: 10.3389/fpls.2016.01431. eCollection 2016. Front Plant Sci. 2016. PMID: 27746789 Free PMC article.
-
Identification of molecular markers associated with Verticillium wilt resistance in alfalfa (Medicago sativa L.) using high-resolution melting.PLoS One. 2014 Dec 23;9(12):e115953. doi: 10.1371/journal.pone.0115953. eCollection 2014. PLoS One. 2014. PMID: 25536106 Free PMC article.
-
Annual Medicago: from a model crop challenged by a spectrum of necrotrophic pathogens to a model plant to explore the nature of disease resistance.Ann Bot. 2006 Dec;98(6):1117-28. doi: 10.1093/aob/mcl132. Epub 2006 Jun 27. Ann Bot. 2006. PMID: 16803846 Free PMC article. Review.
Cited by
-
Optimizing greenhouse microclimate for plant pathology: challenges and cooling solutions for pathogen control in arid regions.Front Plant Sci. 2025 Feb 6;16:1492760. doi: 10.3389/fpls.2025.1492760. eCollection 2025. Front Plant Sci. 2025. PMID: 39980477 Free PMC article. Review.
-
Functional Evolution of Pseudofabraea citricarpa as an Adaptation to Temperature Change.J Fungi (Basel). 2024 Jan 28;10(2):109. doi: 10.3390/jof10020109. J Fungi (Basel). 2024. PMID: 38392781 Free PMC article.
-
Medicago truncatula quantitative resistance to a new strain of Verticillium alfalfae from Iran revealed by a genome-wide association study.Front Plant Sci. 2023 Apr 14;14:1125551. doi: 10.3389/fpls.2023.1125551. eCollection 2023. Front Plant Sci. 2023. PMID: 37123855 Free PMC article.
References
-
- Acharya S. N., Huang H.-C. (2003). Breeding alfalfa for resistance to verticillium wilt: A sound strategy, in Huang H., Acharya S. (Eds.) Advances in Plant Disease Management. (Trivandrum, India: Research Signpost; ) 345–371.
-
- Agresti A. (2012). Analysis of ordinal categorical data (Hoboken, New Jersey, U.S.A.:Wiley; ).
-
- Agrios G. N. (2005). Plant pathology (Amsterdam, NL: Elsevier Science; ).
LinkOut - more resources
Full Text Sources
Miscellaneous

