Pyruvate dehydrogenase kinase regulates vascular inflammation in atherosclerosis and increases cardiovascular risk
- PMID: 36866436
- PMCID: PMC10318388
- DOI: 10.1093/cvr/cvad038
Pyruvate dehydrogenase kinase regulates vascular inflammation in atherosclerosis and increases cardiovascular risk
Abstract
Aims: Recent studies have revealed a close connection between cellular metabolism and the chronic inflammatory process of atherosclerosis. While the link between systemic metabolism and atherosclerosis is well established, the implications of altered metabolism in the artery wall are less understood. Pyruvate dehydrogenase kinase (PDK)-dependent inhibition of pyruvate dehydrogenase (PDH) has been identified as a major metabolic step regulating inflammation. Whether the PDK/PDH axis plays a role in vascular inflammation and atherosclerotic cardiovascular disease remains unclear.
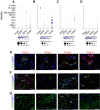
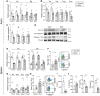
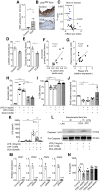
Methods and results: Gene profiling of human atherosclerotic plaques revealed a strong correlation between PDK1 and PDK4 transcript levels and the expression of pro-inflammatory and destabilizing genes. Remarkably, the PDK1 and PDK4 expression correlated with a more vulnerable plaque phenotype, and PDK1 expression was found to predict future major adverse cardiovascular events. Using the small-molecule PDK inhibitor dichloroacetate (DCA) that restores arterial PDH activity, we demonstrated that the PDK/PDH axis is a major immunometabolic pathway, regulating immune cell polarization, plaque development, and fibrous cap formation in Apoe-/- mice. Surprisingly, we discovered that DCA regulates succinate release and mitigates its GPR91-dependent signals promoting NLRP3 inflammasome activation and IL-1β secretion by macrophages in the plaque.
Conclusions: We have demonstrated for the first time that the PDK/PDH axis is associated with vascular inflammation in humans and particularly that the PDK1 isozyme is associated with more severe disease and could predict secondary cardiovascular events. Moreover, we demonstrate that targeting the PDK/PDH axis with DCA skews the immune system, inhibits vascular inflammation and atherogenesis, and promotes plaque stability features in Apoe-/- mice. These results point toward a promising treatment to combat atherosclerosis.
Keywords: CVD; PDK; atherosclerosis; immunometabolism; inflammation.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: I.K. reports personal fees from Orion Pharma unrelated to the submitted work. T.W.S. is a co-founder of Embark Biotech; T.W.S. and M.T. are co-founders of SOLID Therapeutics; unrelated to the submitted work. The remaining authors have nothing to disclose.
Figures



References
-
- Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 2014;35:2950–2959. - PubMed
-
- Ketelhuth DF, Hansson GK. Modulation of autoimmunity and atherosclerosis—common targets and promising translational approaches against disease. Circ J 2015;79:924–933. - PubMed
-
- Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol 2017;18:488–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

