Tissue-mimetic culture enhances mesenchymal stem cell secretome capacity to improve regenerative activity of keratinocytes and fibroblasts in vitro
- PMID: 36866522
- PMCID: PMC12312442
- DOI: 10.1111/wrr.13076
Tissue-mimetic culture enhances mesenchymal stem cell secretome capacity to improve regenerative activity of keratinocytes and fibroblasts in vitro
Abstract
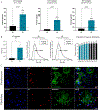
Mesenchymal stem/stromal cells (MSCs) are a heterogenous population of multipotent and highly secretory cells currently being investigated in the field of wound healing for their ability to augment tissue responses. The adaptive response of MSC populations to the rigid substrate of current 2D culture systems has been considered to result in a deterioration of regenerative 'stem-like' properties. In this study, we characterise how the improved culture of adipose-derived mesenchymal stem cells (ASCs) within a tissue-mimetic 3D hydrogel system, that is mechanically similar to native adipose tissue, enhances their regenerative capabilities. Notably, the hydrogel system contains a porous microarchitecture that permits mass transport, enabling efficient collection of secreted cellular compounds. By utilising this 3D system, ASCs retained a significantly higher expression of ASC 'stem-like' markers while demonstrating a significant reduction in senescent populations, relative to 2D. Additionally, culture of ASCs within the 3D system resulted in enhanced secretory activity with significant increases in the secretion of proteinaceous factors, antioxidants and extracellular vesicles (EVs) within the conditioned media (CM) fraction. Lastly, treatment of wound healing cells, keratinocytes (KCs) and fibroblasts (FBs), with ASC-CM from the 2D and 3D systems resulted in augmented functional regenerative activity, with ASC-CM from the 3D system significantly increasing KC and FB metabolic, proliferative and migratory activity. This study demonstrates the potential beneficial role of MSC culture within a tissue-mimetic 3D hydrogel system that more closely mimics native tissue mechanics, and subsequently how the improved phenotype augments secretory activity and potential wound healing capabilities of the MSC secretome.
Keywords: biomaterials; hydrogels; regenerative medicine; secretome; stem cells; tissue engineering.
© 2023 The Authors. Wound Repair and Regeneration published by Wiley Periodicals LLC on behalf of The Wound Healing Society.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
All authors declare that they do not have any conflict of interest except Heather Decker and Dr. Adam Mellott. Heather Decker and Dr. Mellott declare that they have financial interest with Ronawk, which is the company that produces and donated the T-Blocks used in this study. Heather Decker is the co-founder and CTO and Dr. Mellott is the co-founder and CEO of Ronawk.
Figures

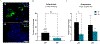

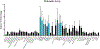
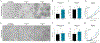
Similar articles
-
Advancing wound healing by hydrogel-based dressings loaded with cell-conditioned medium: a systematic review.Stem Cell Res Ther. 2024 Oct 17;15(1):371. doi: 10.1186/s13287-024-03976-x. Stem Cell Res Ther. 2024. PMID: 39420416 Free PMC article.
-
Adipose-Derived Stem Cell Conditioned Medium and Wound Healing: A Systematic Review.Tissue Eng Part B Rev. 2022 Aug;28(4):830-847. doi: 10.1089/ten.TEB.2021.0100. Epub 2022 Jan 10. Tissue Eng Part B Rev. 2022. PMID: 34409890
-
Ultrafiltration and Injection of Islet Regenerative Stimuli Secreted by Pancreatic Mesenchymal Stromal Cells.Stem Cells Dev. 2021 Mar;30(5):247-264. doi: 10.1089/scd.2020.0206. Epub 2021 Feb 19. Stem Cells Dev. 2021. PMID: 33403929 Free PMC article.
-
Culture of Hoffa fat pad mesenchymal stem/stromal cells on microcarrier suspension in vertical wheel bioreactor for extracellular vesicle production.Stem Cell Res Ther. 2024 Mar 5;15(1):61. doi: 10.1186/s13287-024-03681-9. Stem Cell Res Ther. 2024. PMID: 38439108 Free PMC article.
-
Cell Sheets Formation Enhances Therapeutic Effects of Human Umbilical Cord Mesenchymal Stem Cells on Spinal Cord Injury.CNS Neurosci Ther. 2024 Dec;30(12):e70163. doi: 10.1111/cns.70163. CNS Neurosci Ther. 2024. PMID: 39670537 Free PMC article.
Cited by
-
The Hidden Power of the Secretome: Therapeutic Potential on Wound Healing and Cell-Free Regenerative Medicine-A Systematic Review.Int J Mol Sci. 2025 Feb 23;26(5):1926. doi: 10.3390/ijms26051926. Int J Mol Sci. 2025. PMID: 40076553 Free PMC article.
-
Biomaterials for immunomodulation in wound healing.Regen Biomater. 2024 Mar 27;11:rbae032. doi: 10.1093/rb/rbae032. eCollection 2024. Regen Biomater. 2024. PMID: 38779347 Free PMC article. Review.
-
Stem cell-derived small extracellular vesicles embedded into methacrylated hyaluronic acid wound dressings accelerate wound repair in a pressure model of diabetic ulcer.J Nanobiotechnology. 2023 Dec 7;21(1):469. doi: 10.1186/s12951-023-02202-9. J Nanobiotechnology. 2023. PMID: 38062461 Free PMC article.
-
Advancing wound healing by hydrogel-based dressings loaded with cell-conditioned medium: a systematic review.Stem Cell Res Ther. 2024 Oct 17;15(1):371. doi: 10.1186/s13287-024-03976-x. Stem Cell Res Ther. 2024. PMID: 39420416 Free PMC article.
-
Mesenchymal Stem Cell Extracellular Vesicles from Tissue-Mimetic System Enhance Epidermal Regeneration via Formation of Migratory Cell Sheets.Tissue Eng Regen Med. 2023 Oct;20(6):993-1013. doi: 10.1007/s13770-023-00565-6. Epub 2023 Jul 29. Tissue Eng Regen Med. 2023. PMID: 37515738 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

