PD‑1/PD‑L1 immune checkpoint inhibitors in neoadjuvant therapy for solid tumors (Review)
- PMID: 36866750
- PMCID: PMC10019757
- DOI: 10.3892/ijo.2023.5497
PD‑1/PD‑L1 immune checkpoint inhibitors in neoadjuvant therapy for solid tumors (Review)
Abstract
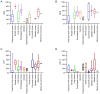
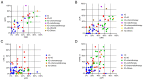
A comprehensive search regarding programmed cell death protein 1 (PD‑1)/programmed death‑ligand 1 (PD‑L1) inhibitor monotherapy or combination therapy in neoadjuvant settings of 11 types of solid cancer was performed using the PubMed, Cochrane and Embase databases, and the abstracts of various conferences were screened. Data presented in 99 clinical trials indicated that preoperative treatment with PD‑1/PD‑L1 combined therapy, particularly immunotherapy plus chemotherapy, could achieve a higher objective response rate, a higher major pathologic response rate and a higher pathologic complete response rate, as well as a lower number of immune‑related adverse events compared with PD‑1/PD‑L1 monotherapy or dual immunotherapy. Although PD‑1/PD‑L1 inhibitor combination caused more treatment‑related adverse events (TRAEs) in patients, most of the TRAEs were acceptable and did not cause marked delays in operation. The data suggest that patients with pathological remission after neoadjuvant immunotherapy exhibit improved postoperative disease‑free survival compared with those without pathological remission. Further studies are still required to evaluate the long‑term survival benefit of neoadjuvant immunotherapy.
Keywords: MPR; ORR; clinical trials; neoadjuvant therapy; pCR; programmed cell death protein 1/programmed death‑ligand 1; solid tumor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, et al. Five-Year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 Study. J Clin Oncol. 2019;37:2518–2527. doi: 10.1200/JCO.19.00934. - DOI - PMC - PubMed
-
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. - DOI - PMC - PubMed
-
- Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
