Nanoparticle-Based Chimeric Antigen Receptor Therapy for Cancer Immunotherapy
- PMID: 36867402
- PMCID: PMC9983528
- DOI: 10.1007/s13770-022-00515-8
Nanoparticle-Based Chimeric Antigen Receptor Therapy for Cancer Immunotherapy
Abstract
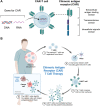
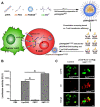
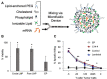
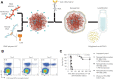
Adoptive cell therapy with chimeric antigen receptor (CAR)-engineered T cells (CAR-Ts) has emerged as an innovative immunotherapy for hematological cancer treatment. However, the limited effect on solid tumors, complex processes, and excessive manufacturing costs remain as limitations of CAR-T therapy. Nanotechnology provides an alternative to the conventional CAR-T therapy. Owing to their unique physicochemical properties, nanoparticles can not only serve as a delivery platform for drugs but also target specific cells. Nanoparticle-based CAR therapy can be applied not only to T cells but also to CAR-natural killer and CAR-macrophage, compensating for some of their limitations. This review focuses on the introduction of nanoparticle-based advanced CAR immune cell therapy and future perspectives on immune cell reprogramming.
Keywords: Cancer immunotherapy; Chimeric antigen receptor (CAR); Genetic engineering; Immune cell reprograming; Nanoparticle.
© 2023. Korean Tissue Engineering and Regenerative Medicine Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
