Endothelial mechanisms for inactivation of inflammation-induced hyperpermeability
- PMID: 36867447
- PMCID: PMC10069978
- DOI: 10.1152/ajpheart.00543.2022
Endothelial mechanisms for inactivation of inflammation-induced hyperpermeability
Abstract
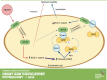
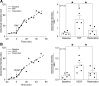
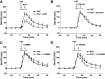
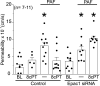
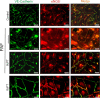
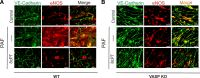
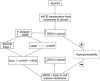
Microvascular hyperpermeability is a hallmark of inflammation. Many negative effects of hyperpermeability are due to its persistence beyond what is required for preserving organ function. Therefore, we propose that targeted therapeutic approaches focusing on mechanisms that terminate hyperpermeability would avoid the negative effects of prolonged hyperpermeability while retaining its short-term beneficial effects. We tested the hypothesis that inflammatory agonist signaling leads to hyperpermeability and initiates a delayed cascade of cAMP-dependent pathways that causes inactivation of hyperpermeability. We applied platelet-activating factor (PAF) and vascular endothelial growth factor (VEGF) to induce hyperpermeability. We used an Epac1 agonist to selectively stimulate exchange protein activated by cAMP (Epac1) and promote inactivation of hyperpermeability. Stimulation of Epac1 inactivated agonist-induced hyperpermeability in the mouse cremaster muscle and in human microvascular endothelial cells (HMVECs). PAF induced nitric oxide (NO) production and hyperpermeability within 1 min and NO-dependent increased cAMP concentration in about 15-20 min in HMVECs. PAF triggered phosphorylation of vasodilator-stimulated phosphoprotein (VASP) in a NO-dependent manner. Epac1 stimulation promoted cytosol-to-membrane eNOS translocation in HMVECs and in myocardial microvascular endothelial (MyEnd) cells from wild-type mice, but not in MyEnd cells from VASP knockout mice. We demonstrate that PAF and VEGF cause hyperpermeability and stimulate the cAMP/Epac1 pathway to inactivate agonist-induced endothelial/microvascular hyperpermeability. Inactivation involves VASP-assisted translocation of eNOS from the cytosol to the endothelial cell membrane. We demonstrate that hyperpermeability is a self-limiting process, whose timed inactivation is an intrinsic property of the microvascular endothelium that maintains vascular homeostasis in response to inflammatory conditions.NEW & NOTEWORTHY Termination of microvascular hyperpermeability has been so far accepted to be a passive result of the removal of the applied proinflammatory agonists. We provide in vivo and in vitro evidence that 1) inactivation of hyperpermeability is an actively regulated process, 2) proinflammatory agonists (PAF and VEGF) stimulate microvascular hyperpermeability and initiate endothelial mechanisms that terminate hyperpermeability, and 3) eNOS location-translocation is critical in the activation-inactivation cascade of endothelial hyperpermeability.
Keywords: endothelium; hyperpermeability; inflammation; vascular biology.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

