Thalamic control of sensory processing and spindles in a biophysical somatosensory thalamoreticular circuit model of wakefulness and sleep
- PMID: 36867532
- PMCID: PMC10066598
- DOI: 10.1016/j.celrep.2023.112200
Thalamic control of sensory processing and spindles in a biophysical somatosensory thalamoreticular circuit model of wakefulness and sleep
Abstract
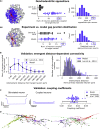
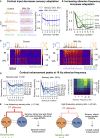
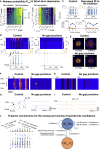
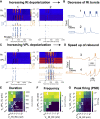
Thalamoreticular circuitry plays a key role in arousal, attention, cognition, and sleep spindles, and is linked to several brain disorders. A detailed computational model of mouse somatosensory thalamus and thalamic reticular nucleus has been developed to capture the properties of over 14,000 neurons connected by 6 million synapses. The model recreates the biological connectivity of these neurons, and simulations of the model reproduce multiple experimental findings in different brain states. The model shows that inhibitory rebound produces frequency-selective enhancement of thalamic responses during wakefulness. We find that thalamic interactions are responsible for the characteristic waxing and waning of spindle oscillations. In addition, we find that changes in thalamic excitability control spindle frequency and their incidence. The model is made openly available to provide a new tool for studying the function and dysfunction of the thalamoreticular circuitry in various brain states.
Keywords: CP: Neuroscience; computer model; sensory processing; sleep; spindles; thalamic reticular nucleus; thalamus; wakefulness.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Jones E.G. Second edition. Cambridge University Press; 2007. The Thalamus. 1939-2011.
-
- Ohara P.T., Lieberman A.R. The thalamic reticular nucleus of the adult rat: experimental anatomical studies. J. Neurocytol. 1985;14:365–411. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

