Tumor suppressor p53 regulates heat shock factor 1 protein degradation in Huntington's disease
- PMID: 36867535
- PMCID: PMC10128052
- DOI: 10.1016/j.celrep.2023.112198
Tumor suppressor p53 regulates heat shock factor 1 protein degradation in Huntington's disease
Abstract
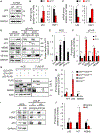
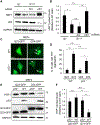
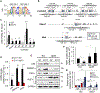
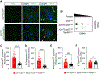
p53 and HSF1 are two major transcription factors involved in cell proliferation and apoptosis, whose dysregulation contributes to cancer and neurodegeneration. Contrary to most cancers, p53 is increased in Huntington's disease (HD) and other neurodegenerative diseases, while HSF1 is decreased. p53 and HSF1 reciprocal regulation has been shown in different contexts, but their connection in neurodegeneration remains understudied. Using cellular and animal models of HD, we show that mutant HTT stabilized p53 by abrogating the interaction between p53 and E3 ligase MDM2. Stabilized p53 promotes protein kinase CK2 alpha prime and E3 ligase FBXW7 transcription, both of which are responsible for HSF1 degradation. Consequently, p53 deletion in striatal neurons of zQ175 HD mice restores HSF1 abundance and decrease HTT aggregation and striatal pathology. Our work shows the mechanism connecting p53 stabilization with HSF1 degradation and pathophysiology in HD and sheds light on the broader molecular differences and commonalities between cancer and neurodegeneration.
Keywords: CP: Molecular biology; CP: Neuroscience; HSF1; Huntington’s disease; MDM2; p53; striatal pathology.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Rosas HD, Lee SY, Bender AC, Zaleta AK, Vangel M, Yu P, Fischl B, Pappu V, Onorato C, Cha JH, et al. (2010). Altered white matter microstructure in the corpus callosum in Huntington’s disease: implications for cortical "disconnection. Neuroimage 49, 2995–3004. 10.1016/j.neuroimage.2009.10.015. - DOI - PMC - PubMed
-
- Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR, Jones R, Landwehrmeyer GB, Fox NC, Johnson H, Hicks SL, et al. (2011). Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol. 10, 31–42. 10.1016/S1474-4422(10)70276-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

