CD169+ macrophage intrinsic IL-10 production regulates immune homeostasis during sepsis
- PMID: 36867536
- PMCID: PMC10123955
- DOI: 10.1016/j.celrep.2023.112171
CD169+ macrophage intrinsic IL-10 production regulates immune homeostasis during sepsis
Abstract
Macrophages facilitate critical functions in regulating pathogen clearance and immune homeostasis in tissues. The remarkable functional diversity exhibited by macrophage subsets is dependent on tissue environment and the nature of the pathological insult. Our current knowledge of the mechanisms that regulate the multifaceted counter-inflammatory responses mediated by macrophages remains incomplete. Here, we report that CD169+ macrophage subsets are necessary for protection under excessive inflammatory conditions. We show that in the absence of these macrophages, even under mild septic conditions, mice fail to survive and exhibit increased production of inflammatory cytokines. Mechanistically, CD169+ macrophages control inflammatory responses via interleukin-10 (IL-10), as CD169+ macrophage-specific deletion of IL-10 was lethal during septic conditions, and recombinant IL-10 treatment reduced lipopolysaccharide (LPS)-induced lethality in mice lacking CD169+ macrophages. Collectively, our findings show a pivotal homeostatic role for CD169+ macrophages and suggest they may serve as an important target for therapy under damaging inflammatory conditions.
Keywords: CD169; CP: Immunology; G-CSF; IL-10; LPS; Siglec-1; macrophages; septic shock.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
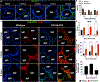
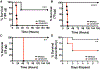




References
-
- Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, and Lecuit M (2015). Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity 42, 145–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

