In-vivo studies on Transitmycin, a potent Mycobacterium tuberculosis inhibitor
- PMID: 36867599
- PMCID: PMC9983862
- DOI: 10.1371/journal.pone.0282454
In-vivo studies on Transitmycin, a potent Mycobacterium tuberculosis inhibitor
Abstract
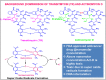
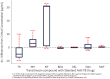
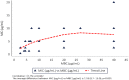
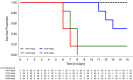
This study involves the in-vitro and in-vivo anti-TB potency and in-vivo safety of Transitmycin (TR) (PubChem CID:90659753)- identified to be a novel secondary metabolite derived from Streptomyces sp (R2). TR was tested in-vitro against drug resistant TB clinical isolates (n = 49). 94% of DR-TB strains (n = 49) were inhibited by TR at 10μg ml-1. In-vivo safety and efficacy studies showed that 0.005mg kg-1 of TR is toxic to mice, rats and guinea pigs, while 0.001mg kg-1 is safe, infection load did not reduce. TR is a potent DNA intercalator and also targets RecA and methionine aminopeptidases of Mycobacterium. Analogue 47 of TR was designed using in-silico based molecule detoxification approaches and SAR analysis. The multiple targeting nature of the TR brightens the chances of the analogues of TR to be a potent TB therapeutic molecule even though the parental compound is toxic. Analog 47 of TR is proposed to have non-DNA intercalating property and lesser in-vivo toxicity with high functional potency. This study attempts to develop a novel anti-TB molecule from microbial sources. Though the parental compound is toxic, its analogs are designed to be safe through in-silico approaches. However, further laboratory validations on this claim need to be carried out before labelling it as a promising anti-TB molecule.
Copyright: © 2023 Mondal et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Authors have declared that no competing interests exist.
Figures

References
-
- Iseman MD. Tuberculosis therapy: past, present and future. European Respiratory Journal. 2002;20(36 suppl):87S LP–94s. http://erj.ersjournals.com/content/20/36_suppl/87S.abstract. - PubMed
-
- Pablos-Méndez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, et al.. Global surveillance for antituberculosis-drug resistance, 1994–1997. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. The New England journal of medicine. 1998;338(23):1641–1649. doi: 10.1056/NEJM199806043382301 - DOI - PubMed
-
- Dye C, Espinal MA. Will tuberculosis become resistant to all antibiotics? Proceedings. Biological sciences. 2001;268(1462):45–52. https://pubmed.ncbi.nlm.nih.gov/12123297. doi: 10.1098/rspb.2000.1328 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

