Real-time redox adaptations in human airway epithelial cells exposed to isoprene hydroxy hydroperoxide
- PMID: 36867944
- PMCID: PMC10011437
- DOI: 10.1016/j.redox.2023.102646
Real-time redox adaptations in human airway epithelial cells exposed to isoprene hydroxy hydroperoxide
Abstract
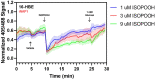
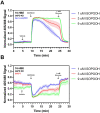
While redox processes play a vital role in maintaining intracellular homeostasis by regulating critical signaling and metabolic pathways, supra-physiological or sustained oxidative stress can lead to adverse responses or cytotoxicity. Inhalation of ambient air pollutants such as particulate matter and secondary organic aerosols (SOA) induces oxidative stress in the respiratory tract through mechanisms that remain poorly understood. We investigated the effect of isoprene hydroxy hydroperoxide (ISOPOOH), an atmospheric oxidation product of vegetation-derived isoprene and a constituent of SOA, on intracellular redox homeostasis in cultured human airway epithelial cells (HAEC). We used high-resolution live cell imaging of HAEC expressing the genetically encoded ratiometric biosensors Grx1-roGFP2, iNAP1, or HyPer, to assess changes in the cytoplasmic ratio of oxidized glutathione to reduced glutathione (GSSG:GSH), and the flux of NADPH and H2O2, respectively. Non-cytotoxic exposure to ISOPOOH resulted in a dose-dependent increase of GSSG:GSH in HAEC that was markedly potentiated by prior glucose deprivation. ISOPOOH-induced increase in glutathione oxidation were accompanied by concomitant decreases in intracellular NADPH. Following ISOPOOH exposure, the introduction of glucose resulted in a rapid restoration of GSH and NADPH, while the glucose analog 2-deoxyglucose resulted in inefficient restoration of baseline GSH and NADPH. To elucidate bioenergetic adaptations involved in combatting ISOPOOH-induced oxidative stress we investigated the regulatory role of glucose-6-phosphate dehydrogenase (G6PD). A knockout of G6PD markedly impaired glucose-mediated recovery of GSSG:GSH but not NADPH. These findings reveal rapid redox adaptations involved in the cellular response to ISOPOOH and provide a live view of the dynamic regulation of redox homeostasis in human airway cells as they are exposed to environmental oxidants.
Keywords: Air pollution; Glutathione; Isoprene hydroxy hydroperoxide; Live cell imaging; NADPH; Oxidative stress.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
GAPDH inhibition mediated by thiol oxidation in human airway epithelial cells exposed to an environmental peroxide.Redox Biol. 2024 Jul;73:103199. doi: 10.1016/j.redox.2024.103199. Epub 2024 May 17. Redox Biol. 2024. PMID: 38810423 Free PMC article.
-
Live cell imaging of oxidative stress in human airway epithelial cells exposed to isoprene hydroxyhydroperoxide.Redox Biol. 2022 May;51:102281. doi: 10.1016/j.redox.2022.102281. Epub 2022 Mar 15. Redox Biol. 2022. PMID: 35306372 Free PMC article.
-
Understanding the Early Biological Effects of Isoprene-Derived Particulate Matter Enhanced by Anthropogenic Pollutants.Res Rep Health Eff Inst. 2019 Mar;2019(198):1-54. Res Rep Health Eff Inst. 2019. PMID: 31872748 Free PMC article.
-
Protein S-glutathionlyation links energy metabolism to redox signaling in mitochondria.Redox Biol. 2016 Aug;8:110-8. doi: 10.1016/j.redox.2015.12.010. Epub 2015 Dec 31. Redox Biol. 2016. PMID: 26773874 Free PMC article. Review.
-
Lighting the light reactions of photosynthesis by means of redox-responsive genetically encoded biosensors for photosynthetic intermediates.Photochem Photobiol Sci. 2023 Aug;22(8):2005-2018. doi: 10.1007/s43630-023-00425-1. Epub 2023 May 17. Photochem Photobiol Sci. 2023. PMID: 37195389 Review.
Cited by
-
Targeting novel regulated cell death: disulfidptosis in cancer immunotherapy with immune checkpoint inhibitors.Biomark Res. 2025 Feb 26;13(1):35. doi: 10.1186/s40364-025-00748-4. Biomark Res. 2025. PMID: 40012016 Free PMC article. Review.
-
Monitoring redox stress in human airway epithelial cells exposed to woodsmoke at an air-liquid interface.Part Fibre Toxicol. 2024 Mar 8;21(1):14. doi: 10.1186/s12989-024-00575-9. Part Fibre Toxicol. 2024. PMID: 38459567 Free PMC article.
-
GAPDH inhibition mediated by thiol oxidation in human airway epithelial cells exposed to an environmental peroxide.Redox Biol. 2024 Jul;73:103199. doi: 10.1016/j.redox.2024.103199. Epub 2024 May 17. Redox Biol. 2024. PMID: 38810423 Free PMC article.
References
-
- Burnett R.T., Pope C.A., Ezzati M., Olives C., Lim S.S., Mehta S., Shin H.H., Singh G., Hubbell B., Brauer M., et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ. Health Perspect. 2014;122:397–403. - PMC - PubMed
-
- Samet J.M., Zeger S.L., Dominici F., Curriero F., Coursac I., Dockery D.W., Schwartz J., Zanobetti A. The national morbidity, mortality, and air pollution study. Part II: morbidity and mortality from air pollution in the United States. Res. Rep. Health Eff. Inst. 2000;94:5–70. - PubMed
-
- Arashiro M., Lin Y.H., Zhang Z., Sexton K.G., Gold A., Jaspers I., Fry R.C., Surratt J.D. Effect of secondary organic aerosol from isoprene-derived hydroxyhydroperoxides on the expression of oxidative stress response genes in human bronchial epithelial cells. Environ. Sci. Process Impacts. 2018;20:332–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

