Sustained alternate-day fasting potentiates doxorubicin cardiotoxicity
- PMID: 36868222
- PMCID: PMC10257771
- DOI: 10.1016/j.cmet.2023.02.006
Sustained alternate-day fasting potentiates doxorubicin cardiotoxicity
Abstract
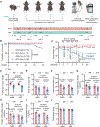
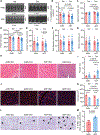
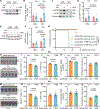
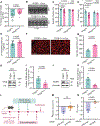
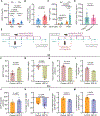
Fasting strategies are under active clinical investigation in patients receiving chemotherapy. Prior murine studies suggest that alternate-day fasting may attenuate doxorubicin cardiotoxicity and stimulate nuclear translocation of transcription factor EB (TFEB), a master regulator of autophagy and lysosomal biogenesis. In this study, human heart tissue from patients with doxorubicin-induced heart failure demonstrated increased nuclear TFEB protein. In mice treated with doxorubicin, alternate-day fasting or viral TFEB transduction increased mortality and impaired cardiac function. Mice randomized to alternate-day fasting plus doxorubicin exhibited increased TFEB nuclear translocation in the myocardium. When combined with doxorubicin, cardiomyocyte-specific TFEB overexpression provoked cardiac remodeling, while systemic TFEB overexpression increased growth differentiation factor 15 (GDF15) and caused heart failure and death. Cardiomyocyte TFEB knockout attenuated doxorubicin cardiotoxicity, while recombinant GDF15 was sufficient to cause cardiac atrophy. Our studies identify that both sustained alternate-day fasting and a TFEB/GDF15 pathway exacerbate doxorubicin cardiotoxicity.
Keywords: TFEB; cardiotoxicity; doxorubicin; intermittent fasting.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.J. has a pending patent for fusion protein nanodiscs for the treatment of heart failure and eye disease, is a member of the scientific advisory board of Mobius Scientific, and receives research funding from AstraZeneca, unrelated to the studies in this manuscript. M.K. receives consulting fees/honoraria from AstraZeneca, Amgen, Sanofi-Aventis, Boehringer Ingelheim, Glytec, Merck, Janssen Pharmaceuticals, Novartis, Applied Therapeutics, Bayer Healthcare Pharmaceuticals, Eli Lilly and Company, and Vifor Pharma and research grants from AstraZeneca and Boehringer Ingelheim. A. Diwan reports consulting for clinical trials with Clario (previously ERT/Biomedical systems) and serves on the scientific advisory board for Dewpoint Therapeutics, which are not relevant to the current study.
Figures
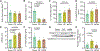

Comment in
-
Unexpected worsening of doxorubicin cardiotoxicity upon intermittent fasting.Med. 2023 May 12;4(5):288-289. doi: 10.1016/j.medj.2023.04.005. Med. 2023. PMID: 37178681
-
Alternate fasting potentiates doxorubicin-induced cardiotoxicity.Nat Cardiovasc Res. 2023 May;2(5):418. doi: 10.1038/s44161-023-00275-1. Nat Cardiovasc Res. 2023. PMID: 39196049 No abstract available.
References
-
- Ma X, Mani K, Liu H, Kovacs A, Murphy JT, Foroughi L, French BA, Weinheimer CJ, Kraja A, Benjamin IJ, et al. (2019). Transcription Factor EB Activation Rescues Advanced alphaB-Crystallin Mutation-Induced Cardiomyopathy by Normalizing Desmin Localization. J Am Heart Assoc 8, e010866. 10.1161/JAHA.118.010866. - DOI - PMC - PubMed
-
- Liu H, Javaheri A, Godar RJ, Murphy J, Ma X, Rohatgi N, Mahadevan J, Hyrc K, Saftig P, Marshall C, et al. (2017). Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy 13, 1952–1968. 10.1080/15548627.2017.1368596. - DOI - PMC - PubMed
-
- Godar RJ, Ma X, Liu H, Murphy JT, Weinheimer CJ, Kovacs A, Crosby SD, Saftig P, and Diwan A (2015). Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury. Autophagy 11, 1537–1560. 10.1080/15548627.2015.1063768. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL159461/HL/NHLBI NIH HHS/United States
- R01 HL143431/HL/NHLBI NIH HHS/United States
- R01 HL107594/HL/NHLBI NIH HHS/United States
- R01 HL147884/HL/NHLBI NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- I01 BX005981/BX/BLRD VA/United States
- K08 HL163469/HL/NHLBI NIH HHS/United States
- R01 DK131188/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- I01 BX003415/BX/BLRD VA/United States
- K08 HL145019/HL/NHLBI NIH HHS/United States
- R01 HL125838/HL/NHLBI NIH HHS/United States
- R01 NS094692/NS/NINDS NIH HHS/United States
- R01 HL155344/HL/NHLBI NIH HHS/United States
- K08 HL138262/HL/NHLBI NIH HHS/United States
- I01 BX004235/BX/BLRD VA/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- S10 OD028597/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

