DAXX adds a de novo H3.3K9me3 deposition pathway to the histone chaperone network
- PMID: 36868228
- PMCID: PMC10114496
- DOI: 10.1016/j.molcel.2023.02.009
DAXX adds a de novo H3.3K9me3 deposition pathway to the histone chaperone network
Abstract
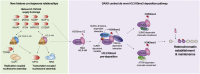
A multitude of histone chaperones are required to support histones from their biosynthesis until DNA deposition. They cooperate through the formation of histone co-chaperone complexes, but the crosstalk between nucleosome assembly pathways remains enigmatic. Using exploratory interactomics, we define the interplay between human histone H3-H4 chaperones in the histone chaperone network. We identify previously uncharacterized histone-dependent complexes and predict the structure of the ASF1 and SPT2 co-chaperone complex, expanding the role of ASF1 in histone dynamics. We show that DAXX provides a unique functionality to the histone chaperone network, recruiting histone methyltransferases to promote H3K9me3 catalysis on new histone H3.3-H4 prior to deposition onto DNA. Hereby, DAXX provides a molecular mechanism for de novo H3K9me3 deposition and heterochromatin assembly. Collectively, our findings provide a framework for understanding how cells orchestrate histone supply and employ targeted deposition of modified histones to underpin specialized chromatin states.
Keywords: ASF1; DAXX; HJURP; NASP; epigenetic; gene silencing; heterochromatin; histone chaperone; nucleosome assembly; protein network; proteomics.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.M.H. and A.G. are inventors on a patent covering the therapeutic targeting of TONSL for cancer therapy. A.G. is co-founder and chief scientific officer of Ankrin Therapeutics. A.G. is a member of Molecular Cell’s Scientific Advisory Board. A.I. and M.V.-A. are cofounders of EpiQMAx. G.M. is co-founder and member of the board of directors of Twelve Bio and is a member of the Scientific Advisory Board at Ensoma.
Figures





Comment in
-
Histone chaperones: A multinodal highway network inside the cell.Mol Cell. 2023 Apr 6;83(7):1024-1026. doi: 10.1016/j.molcel.2023.03.004. Mol Cell. 2023. PMID: 37028413 Free PMC article.
-
Histone H3 and its chaperones.Nat Struct Mol Biol. 2023 Apr;30(4):405. doi: 10.1038/s41594-023-00976-y. Nat Struct Mol Biol. 2023. PMID: 37045977 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

