Efficacy of SARS-CoV-2 vaccines and the dose-response relationship with three major antibodies: a systematic review and meta-analysis of randomised controlled trials
- PMID: 36868258
- PMCID: PMC9974155
- DOI: 10.1016/S2666-5247(22)00390-1
Efficacy of SARS-CoV-2 vaccines and the dose-response relationship with three major antibodies: a systematic review and meta-analysis of randomised controlled trials
Abstract
Background: The efficacy of SARS-CoV-2 vaccines in preventing severe COVID-19 illness and death is uncertain due to the rarity of data in individual trials. How well the antibody concentrations can predict the efficacy is also uncertain. We aimed to assess the efficacy of these vaccines in preventing SARS-CoV-2 infections of different severities and the dose-response relationship between the antibody concentrations and efficacy.
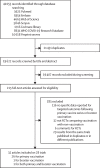
Methods: We did a systematic review and meta-analysis of randomised controlled trials (RCTs). We searched PubMed, Embase, Scopus, Web of Science, Cochrane Library, WHO, bioRxiv, and medRxiv for papers published between Jan 1, 2020 and Sep 12, 2022. RCTs on the efficacy of SARS-CoV-2 vaccines were eligible. Risk of bias was assessed using the Cochrane tool. A frequentist, random-effects model was used to combine efficacy for common outcomes (ie, symptomatic and asymptomatic infections) and a Bayesian random-effects model was used for rare outcomes (ie, hospital admission, severe infection, and death). Potential sources of heterogeneity were investigated. The dose-response relationships of neutralising, spike-specific IgG and receptor binding domain-specific IgG antibody titres with efficacy in preventing SARS-CoV-2 symptomatic and severe infections were examined by meta-regression. This systematic review is registered with PROSPERO, CRD42021287238.
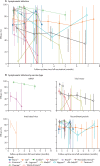
Findings: 28 RCTs (n=286 915 in vaccination groups and n=233 236 in placebo groups; median follow-up 1-6 months after last vaccination) across 32 publications were included in this review. The combined efficacy of full vaccination was 44·5% (95% CI 27·8-57·4) for preventing asymptomatic infections, 76·5% (69·8-81·7) for preventing symptomatic infections, 95·4% (95% credible interval 88·0-98·7) for preventing hospitalisation, 90·8% (85·5-95·1) for preventing severe infection, and 85·8% (68·7-94·6) for preventing death. There was heterogeneity in the efficacy of SARS-CoV-2 vaccines against asymptomatic and symptomatic infections but insufficient evidence to suggest whether the efficacy could differ according to the type of vaccine, age of the vaccinated individual, and between-dose interval (p>0·05 for all). Vaccine efficacy against symptomatic infection waned over time after full vaccination, with an average decrease of 13·6% (95% CI 5·5-22·3; p=0·0007) per month but can be enhanced by a booster. We found a significant non-linear relationship between each type of antibody and efficacy against symptomatic and severe infections (p<0·0001 for all), but there remained considerable heterogeneity in the efficacy, which cannot be explained by antibody concentrations. The risk of bias was low in most studies.
Interpretation: The efficacy of SARS-CoV-2 vaccines is higher for preventing severe infection and death than for preventing milder infection. Vaccine efficacy wanes over time but can be enhanced by a booster. Higher antibody titres are associated with higher estimates of efficacy but precise predictions are difficult due to large unexplained heterogeneity. These findings provide an important knowledge base for interpretation and application of future studies on these issues.
Funding: Shenzhen Science and Technology Programs.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

