Vaccination from the early second trimester onwards gives a robust SARS-CoV-2 antibody response throughout pregnancy and provides antibodies for the neonate
- PMID: 36868302
- PMCID: PMC9977072
- DOI: 10.1016/j.ijid.2023.02.022
Vaccination from the early second trimester onwards gives a robust SARS-CoV-2 antibody response throughout pregnancy and provides antibodies for the neonate
Abstract
Objectives: Preventive measures against COVID-19 are essential for pregnant women. Pregnant women are particularly vulnerable to emerging infectious pathogens due to alterations in their physiology. We aimed to determine the optimum timing of vaccination to protect pregnant women and their neonates from COVID-19.
Methods: A prospective observational longitudinal cohort study in pregnant women who received COVID-19 vaccination. We collected blood samples to evaluate levels of antispike, receptor binding domain and nucleocapsid antibodies against SARS-CoV-2 before vaccination and 15 days after the first and second vaccination. We determined the neutralizing antibodies from mother-infant dyads in maternal and umbilical cord blood at birth. If available, immunoglobulin A was measured in human milk.
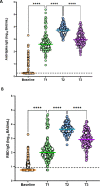
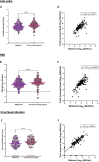
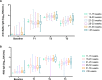
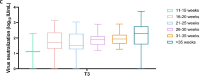
Results: We included 178 pregnant women. Median antispike immunoglobulin G levels increased significantly from 1.8 to 5431 binding antibody units/ml and receptor binding domain from 6 to 4466 binding antibody units/ml. Virus neutralization showed similar results between different weeks of gestation at vaccination (P >0.3).
Conclusion: We advise vaccination in the early second trimester of pregnancy for the optimum balance between the maternal antibody response and placental antibody transfer to the neonate.
Keywords: Antispike IgG; COVID-19; Placental antibody transfer; Pregnancy; Vaccination; Virus neutralization.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no competing interests to declare.
Figures

References
-
- Collin J, Byström E, Carnahan A, Ahrne M. Public Health Agency of Sweden's Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99:819–822. doi: 10.1111/aogs.13901. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

