NEF-Induced HIV-Associated Nephropathy Through HCK/LYN Tyrosine Kinases
- PMID: 36868467
- PMCID: PMC10284032
- DOI: 10.1016/j.ajpath.2023.02.006
NEF-Induced HIV-Associated Nephropathy Through HCK/LYN Tyrosine Kinases
Abstract
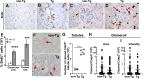
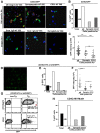
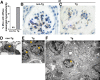
HIV-1-associated nephropathy (HIVAN) is a severe complication of HIV-1 infection. To gain insight into the pathogenesis of kidney disease in the setting of HIV, a transgenic (Tg) mouse model [CD4C/HIV-negative regulator factor (Nef)] was used in which HIV-1 nef expression is under control of regulatory sequences (CD4C) of the human CD4 gene, thus allowing expression in target cells of the virus. These Tg mice develop a collapsing focal segmental glomerulosclerosis associated with microcystic dilatation, similar to human HIVAN. To identify kidney cells permissive to the CD4C promoter, CD4C reporter Tg lines were used. They showed preferential expression in glomeruli, mainly in mesangial cells. Breeding CD4C/HIV Tg mice on 10 different mouse backgrounds showed that HIVAN was modulated by host genetic factors. Studies of gene-deficient Tg mice revealed that the presence of B and T cells and that of several genes was dispensable for the development of HIVAN: those involved in apoptosis (Trp53, Tnfsf10, Tnf, Tnfrsf1b, and Bax), in immune cell recruitment (Ccl3, Ccl2, Ccr2, Ccr5, and Cx3cr1), in nitric oxide (NO) formation (Nos3 and Nos2), or in cell signaling (Fyn, Lck, and Hck/Fgr). However, deletion of Src partially and that of Hck/Lyn largely abrogated its development. These data suggest that Nef expression in mesangial cells through hematopoietic cell kinase (Hck)/Lck/Yes novel tyrosine kinase (Lyn) represents important cellular and molecular events for the development of HIVAN in these Tg mice.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- D'Agati V., Appel G.B. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18:406–421. - PubMed
-
- Ross M.J., Klotman P.E. HIV-associated nephropathy. AIDS. 2004;18:1089–1099. - PubMed
-
- Alpers C.E., Tsai C.C., Hudkins K.L., Cui Y., Kuller L., Benveniste R.E., Ward J.M., Morton W.R. Focal segmental glomerulosclerosis in primates infected with a simian immunodeficiency virus. Aids Res Hum Retrovir. 1997;13:413–424. - PubMed
-
- Gattone V.H., Tian C., Zhuge W., Sahni M., Narayan O., Stephens E.B. SIV-associated nephropathy in rhesus macaques infected with lymphocyte-tropic SIVmac239. AIDS Res Hum Retroviruses. 1998;14:1163–1180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

