Physiology of a Forgotten Electrolyte-Magnesium Disorders
- PMID: 36868730
- PMCID: PMC10291516
- DOI: 10.1053/j.akdh.2022.12.001
Physiology of a Forgotten Electrolyte-Magnesium Disorders
Abstract
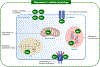
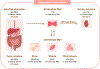
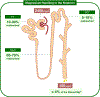
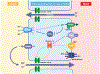
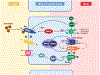
Magnesium (Mg2+) is the second most common intracellular cation and the fourth most abundant element on earth. However, Mg2+ is a frequently overlooked electrolyte and often not measured in patients. While hypomagnesemia is common in 15% of the general population, hypermagnesemia is typically only found in preeclamptic women after Mg2+ therapy and in patients with ESRD. Mild to moderate hypomagnesemia has been associated with hypertension, metabolic syndrome, type 2 diabetes mellitus, CKD, and cancer. Nutritional Mg2+ intake and enteral Mg2+ absorption are important for Mg2+ homeostasis, but the kidneys are the key regulators of Mg2+ homeostasis by limiting urinary excretion to less than 4% while the gastrointestinal tract loses over 50% of the Mg2+ intake in the feces. Here, we review the physiological relevance of Mg2+, the current knowledge of Mg2+ absorption in the kidneys and the gut, the different causes of hypomagnesemia, and a diagnostic approach on how to assess Mg2+ status. We highlight the latest discoveries of monogenetic conditions causing hypomagnesemia, which have enhanced our understanding of tubular Mg2+ absorption. We will also discuss external and iatrogenic causes of hypomagnesemia and advances in the treatment of hypomagnesemia.
Keywords: Distal convoluted tubule; Magnesium; Physiology; Therapies for hypomagnesemia; Thick ascending limb of Henle; Tubule.
Copyright © 2022 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1–46. - PubMed
-
- King DE, Mainous AG 3rd, Geesey ME, Woolson RF. Dietary magnesium and C-reactive protein levels. J Am Coll Nutr. 2005;24(3):166–171. - PubMed
-
- Tangvoraphonkchai K, Davenport A. Magnesium and cardiovascular disease. Adv Chron Kidney Dis. 2018;25(3):251–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

