Physiopathology of Phosphate Disorders
- PMID: 36868732
- PMCID: PMC10565570
- DOI: 10.1053/j.akdh.2022.12.011
Physiopathology of Phosphate Disorders
Abstract
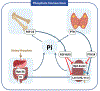
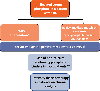
Intracellular phosphate is critical for cellular processes such as signaling, nucleic acid synthesis, and membrane function. Extracellular phosphate (Pi) is an important component of the skeleton. Normal levels of serum phosphate are maintained by the coordinated actions of 1,25-dihydroxyvitamin D3, parathyroid hormone and fibroblast growth factor-23, which intersect in the proximal tubule to control the reabsorption of phosphate via the sodium-phosphate cotransporters Npt2a and Npt2c. Furthermore, 1,25-dihydroxyvitamin D3 participates in the regulation of dietary phosphate absorption in the small intestine. Clinical manifestations associated with abnormal serum phosphate levels are common and occur as a result of genetic or acquired conditions affecting phosphate homeostasis. For example, chronic hypophosphatemia leads to osteomalacia in adults and rickets in children. Acute severe hypophosphatemia can affect multiple organs leading to rhabdomyolysis, respiratory dysfunction, and hemolysis. Patients with impaired kidney function, such as those with advanced CKD, have high prevalence of hyperphosphatemia, with approximately two-thirds of patients on chronic hemodialysis in the United States having serum phosphate levels above the recommended goal of 5.5 mg/dL, a cutoff associated with excess risk of cardiovascular complications. Furthermore, patients with advanced kidney disease and hyperphosphatemia (>6.5 mg/dL) have almost one-third excess risk of death than those with phosphate levels between 2.4 and 6.5 mg/dL. Given the complex mechanisms that regulate phosphate levels, the interventions to treat the various diseases associated with hypophosphatemia or hyperphosphatemia rely on the understanding of the underlying pathobiological mechanisms governing each patient condition.
Copyright © 2023. Published by Elsevier Inc.
Figures

References
-
- Michigami T Advances in understanding of phosphate homeostasis and related disorders. Endocr J. 2022;69(8):881–896. - PubMed
-
- Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ. Phosphorus, phosphorous, and phosphate. Hemodial Int. 2013;17(4):479–482. - PubMed
-
- Bansal VK. Serum inorganic phosphorus. Clinical methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworth Publishers; 1990. Chapter: 198. - PubMed
-
- Christov M, Jüppner H. Phosphate homeostasis disorders. Best Pract Res Clin Endocrinol Metab. 2018;32(5):685–706. - PubMed
-
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

