Selective Serotonin Reuptake Inhibitors within Cells: Temporal Resolution in Cytoplasm, Endoplasmic Reticulum, and Membrane
- PMID: 36868853
- PMCID: PMC10072302
- DOI: 10.1523/JNEUROSCI.1519-22.2022
Selective Serotonin Reuptake Inhibitors within Cells: Temporal Resolution in Cytoplasm, Endoplasmic Reticulum, and Membrane
Abstract
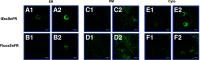
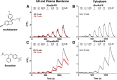
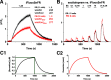
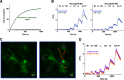
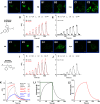
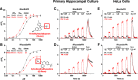
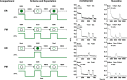
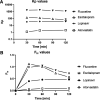
Selective serotonin reuptake inhibitors (SSRIs) are the most prescribed treatment for individuals experiencing major depressive disorder. The therapeutic mechanisms that take place before, during, or after SSRIs bind the serotonin transporter (SERT) are poorly understood, partially because no studies exist on the cellular and subcellular pharmacokinetic properties of SSRIs in living cells. We studied escitalopram and fluoxetine using new intensity-based, drug-sensing fluorescent reporters targeted to the plasma membrane, cytoplasm, or endoplasmic reticulum (ER) of cultured neurons and mammalian cell lines. We also used chemical detection of drug within cells and phospholipid membranes. The drugs attain equilibrium in neuronal cytoplasm and ER at approximately the same concentration as the externally applied solution, with time constants of a few s (escitalopram) or 200-300 s (fluoxetine). Simultaneously, the drugs accumulate within lipid membranes by ≥18-fold (escitalopram) or 180-fold (fluoxetine), and possibly by much larger factors. Both drugs leave cytoplasm, lumen, and membranes just as quickly during washout. We synthesized membrane-impermeant quaternary amine derivatives of the two SSRIs. The quaternary derivatives are substantially excluded from membrane, cytoplasm, and ER for >2.4 h. They inhibit SERT transport-associated currents sixfold or 11-fold less potently than the SSRIs (escitalopram or fluoxetine derivative, respectively), providing useful probes for distinguishing compartmentalized SSRI effects. Although our measurements are orders of magnitude faster than the therapeutic lag of SSRIs, these data suggest that SSRI-SERT interactions within organelles or membranes may play roles during either the therapeutic effects or the antidepressant discontinuation syndrome.SIGNIFICANCE STATEMENT Selective serotonin reuptake inhibitors stabilize mood in several disorders. In general, these drugs bind to SERT, which clears serotonin from CNS and peripheral tissues. SERT ligands are effective and relatively safe; primary care practitioners often prescribe them. However, they have several side effects and require 2-6 weeks of continuous administration until they act effectively. How they work remains perplexing, contrasting with earlier assumptions that the therapeutic mechanism involves SERT inhibition followed by increased extracellular serotonin levels. This study establishes that two SERT ligands, fluoxetine and escitalopram, enter neurons within minutes, while simultaneously accumulating in many membranes. Such knowledge will motivate future research, hopefully revealing where and how SERT ligands engage their therapeutic target(s).
Keywords: biosensor; escitalopram; fluoxetine; iDrugSnFRs; inside-out pharmacology; pharmacokinetics.
Copyright © 2023 the authors.
Figures



References
-
- Alberts B, Bray D, Lewis J, Morgan D, Raff M, Roberts K, Walter P, Wilson J, Hunt TW (2015) Molecular biology of the cell. New York: Garland Science.
-
- Beatty Z, Muthusamy A, unger E, Dougherty DA, Tian L, Looger LL, Shivange AV, Bera K, Lester HA, Nichols A (2022) Fluorescence screens for identifying central nervous system–acting drug-biosensor pairs for subcellular and supracellular pharmacokinetics. Bio Protoc 12:e4551. 10.21769/BioProtoc.4551 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
