A fully human connective tissue growth factor blocking monoclonal antibody ameliorates experimental rheumatoid arthritis through inhibiting angiogenesis
- PMID: 36869335
- PMCID: PMC9985226
- DOI: 10.1186/s12896-023-00776-8
A fully human connective tissue growth factor blocking monoclonal antibody ameliorates experimental rheumatoid arthritis through inhibiting angiogenesis
Abstract
Background: Connective tissue growth factor (CTGF) plays a pivotal role in the pathogenesis of rheumatoid arthritis (RA) by facilitating angiogenesis and is a promising therapeutic target for RA treatment. Herein, we generated a fully human CTGF blocking monoclonal antibody (mAb) through phage display technology.
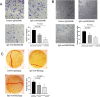
Results: A single-chain fragment variable (scFv) with a high affinity to human CTGF was isolated through screening a fully human phage display library. We carried out affinity maturation to elevate its affinity for CTGF and reconstructed it into a full-length IgG1 format for further optimization. Surface plasmon resonance (SPR) data showed that full-length antibody IgG mut-B2 bound to CTGF with a dissociation constant (KD) as low as 0.782 nM. In the collagen-induced arthritis (CIA) mice, IgG mut-B2 alleviated arthritis and decreased the level of pro-inflammatory cytokines in a dose-dependent manner. Furthermore, we confirmed that the TSP-1 domain of CTGF is essential for the interaction. Additionally, the results of Transwell assays, tube formation experiments, and chorioallantoic membrane (CAM) assays showed that IgG mut-B2 could effectively inhibit angiogenesis.
Conclusion: The fully human mAb that antagonizes CTGF could effectively alleviate arthritis in CIA mice, and its mechanism is tightly associated with the TSP-1 domain of CTGF.
Keywords: Affinity maturation; Angiogenesis; Arthritis; CTGF; Human antibody; Phage display.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Smolen JS, Pangan AL, Emery P, Rigby W, Tanaka Y, Vargas JI, Zhang Y, Damjanov N, Friedman A, Othman AA, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393(10188):2303–11. doi: 10.1016/S0140-6736(19)30419-2. - DOI - PubMed
-
- Huh YH, Lee G, Lee KB, Koh JT, Chun JS, Ryu JH. HIF-2alpha-induced chemokines stimulate motility of fibroblast-like synoviocytes and chondrocytes into the cartilage-pannus interface in experimental rheumatoid arthritis mouse models. Arthritis Res Ther. 2015;17:302. doi: 10.1186/s13075-015-0816-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

