Recent advances of CREKA peptide-based nanoplatforms in biomedical applications
- PMID: 36869341
- PMCID: PMC9985238
- DOI: 10.1186/s12951-023-01827-0
Recent advances of CREKA peptide-based nanoplatforms in biomedical applications
Abstract
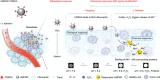
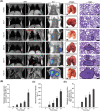
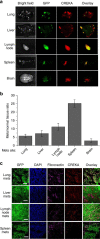
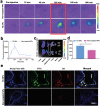
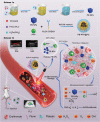
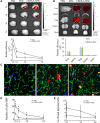
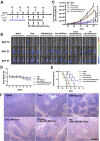
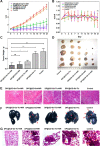
Nanomedicine technology is a rapidly developing field of research and application that uses nanoparticles as a platform to facilitate the diagnosis and treatment of diseases. Nanoparticles loaded with drugs and imaging contrast agents have already been used in clinically, but they are essentially passive delivery carriers. To make nanoparticles smarter, an important function is the ability to actively locate target tissues. It enables nanoparticles to accumulate in target tissues at higher concentrations, thereby improving therapeutic efficacy and reducing side effects. Among the different ligands, the CREKA peptide (Cys-Arg-Glu-Lys-Ala) is a desirable targeting ligand and has a good targeting ability for overexpressed fibrin in different models, such as cancers, myocardial ischemia-reperfusion, and atherosclerosis. In this review, the characteristic of the CREKA peptide and the latest reports regarding the application of CREKA-based nanoplatforms in different biological tissues are described. In addition, the existing problems and future application perspectives of CREKA-based nanoplatforms are also addressed.
Keywords: Active targeting; Biomedical applications; CREKA peptide; Fibrin-fibronectin complexes; Nanoplatform.
© 2023. The Author(s).
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- LQ23H300009/the National Natural Science Foundation of Zhejiang Province
- LQ 22H090015/the National Natural Science Foundation of Zhejiang Province
- 2021ZA010, LGD22H07004/the National Natural Science Foundation of Zhejiang Province
- LGF20H310004/the National Natural Science Foundation of Zhejiang Province
LinkOut - more resources
Full Text Sources
Medical

