PRRC2 proteins impact translation initiation by promoting leaky scanning
- PMID: 36869665
- PMCID: PMC10123092
- DOI: 10.1093/nar/gkad135
PRRC2 proteins impact translation initiation by promoting leaky scanning
Abstract
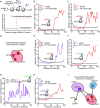
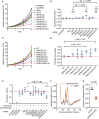
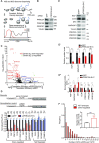
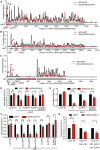
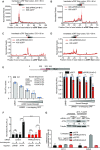
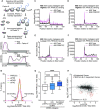
Roughly half of animal mRNAs contain upstream open reading frames (uORFs). These uORFs can represent an impediment to translation of the main ORF since ribosomes usually bind the mRNA cap at the 5' end and then scan for ORFs in a 5'-to-3' fashion. One way for ribosomes to bypass uORFs is via leaky scanning, whereby the ribosome disregards the uORF start codon. Hence leaky scanning is an important instance of post-transcriptional regulation that affects gene expression. Few molecular factors regulating or facilitating this process are known. Here we show that the PRRC2 proteins PRRC2A, PRRC2B and PRRC2C impact translation initiation. We find that they bind eukaryotic translation initiation factors and preinitiation complexes, and are enriched on ribosomes translating mRNAs with uORFs. We find that PRRC2 proteins promote leaky scanning past translation start codons, thereby promoting translation of mRNAs containing uORFs. Since PRRC2 proteins have been associated with cancer, this provides a mechanistic starting point for understanding their physiological and pathophysiological roles.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Translational regulation by uORFs and start codon selection stringency.Genes Dev. 2023 Jun 1;37(11-12):474-489. doi: 10.1101/gad.350752.123. Epub 2023 Jul 11. Genes Dev. 2023. PMID: 37433636 Free PMC article. Review.
-
Translation Complex Profile Sequencing Allows Discrimination of Leaky Scanning and Reinitiation in Upstream Open Reading Frame-controlled Translation.J Mol Biol. 2024 Dec 1;436(23):168850. doi: 10.1016/j.jmb.2024.168850. Epub 2024 Oct 30. J Mol Biol. 2024. PMID: 39486574
-
Leaky scanning and scanning-independent ribosome migration on the tricistronic S1 mRNA of avian reovirus.J Biol Chem. 2007 Aug 31;282(35):25613-22. doi: 10.1074/jbc.M703708200. Epub 2007 Jun 29. J Biol Chem. 2007. PMID: 17604272
-
The Roles of eIF4G2 in Leaky Scanning and Reinitiation on the Human Dual-Coding POLG mRNA.Int J Mol Sci. 2023 Dec 5;24(24):17149. doi: 10.3390/ijms242417149. Int J Mol Sci. 2023. PMID: 38138978 Free PMC article.
-
Non-AUG translation initiation in mammals.Genome Biol. 2022 May 9;23(1):111. doi: 10.1186/s13059-022-02674-2. Genome Biol. 2022. PMID: 35534899 Free PMC article. Review.
Cited by
-
Specific mechanisms of translation initiation in higher eukaryotes: the eIF4G2 story.RNA. 2023 Mar;29(3):282-299. doi: 10.1261/rna.079462.122. Epub 2022 Dec 14. RNA. 2023. PMID: 36517212 Free PMC article. Review.
-
The Prognostic Value of ASPHD1 and ZBTB12 in Colorectal Cancer: A Machine Learning-Based Integrated Bioinformatics Approach.Cancers (Basel). 2023 Aug 28;15(17):4300. doi: 10.3390/cancers15174300. Cancers (Basel). 2023. PMID: 37686578 Free PMC article.
-
Loss-of-function cancer-linked mutations in the EIF4G2 non-canonical translation initiation factor.Life Sci Alliance. 2023 Dec 21;7(3):e202302338. doi: 10.26508/lsa.202302338. Print 2024 Mar. Life Sci Alliance. 2023. PMID: 38129098 Free PMC article.
-
POU4F1 enhances lung cancer gemcitabine resistance by regulating METTL3-dependent TWF1 mRNA N6 adenosine methylation.3 Biotech. 2025 Jan;15(1):7. doi: 10.1007/s13205-024-04161-w. Epub 2024 Dec 12. 3 Biotech. 2025. PMID: 39676891
-
Perturbations in eIF3 subunit stoichiometry alter expression of ribosomal proteins and key components of the MAPK signaling pathways.Elife. 2024 Nov 4;13:RP95846. doi: 10.7554/eLife.95846. Elife. 2024. PMID: 39495207 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

