A Model Template Approach for Rapid Evaluation and Application of Physiologically Based Pharmacokinetic Models: Extension to Volatile Organic Compounds
- PMID: 36869685
- PMCID: PMC11998980
- DOI: 10.1093/toxsci/kfad021
A Model Template Approach for Rapid Evaluation and Application of Physiologically Based Pharmacokinetic Models: Extension to Volatile Organic Compounds
Abstract
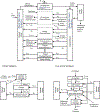
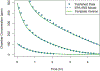
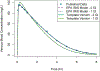
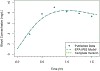
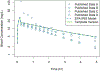
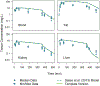
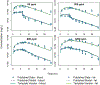
Chemical risk assessors use physiologically based pharmacokinetic (PBPK) models to perform dosimetric calculations, including extrapolations between exposure scenarios, species, and populations of interest. Assessors should complete a thorough quality assurance (QA) review to ensure biological accuracy and correct implementation prior to using these models. This process can be time-consuming, and we developed a PBPK model template that allows for faster, more efficient QA review. The model template consists of a single model "superstructure" with equations and logic commonly found in PBPK models, allowing users to implement a wide variety of chemical-specific PBPK models. QA review can be completed more quickly than for conventional PBPK model implementations because the general model equations have already been reviewed and only parameters describing chemical-specific model and exposure scenarios need review for any given model implementation. We have expanded a previous version of the PBPK model template by adding features commonly included in PBPK models for volatile organic compounds (VOCs). We included multiple options for representing concentrations in blood, describing metabolism, and modeling gas exchange processes to allow for inhalation exposures. We created PBPK model template implementations of published models for seven VOCs: dichloromethane, methanol, chloroform, styrene, vinyl chloride, trichloroethylene, and carbon tetrachloride. Simulations performed using our template implementations matched published simulation results to a high degree of accuracy (maximum observed percent error: 1%). Thus, the model template approach can now be applied to a broader class of chemical-specific PBPK models while continuing to bolster efficiency of QA processes that should be conducted prior to using models for risk assessment applications.
Keywords: PBPK model; VOCs; pharmacokinetics; risk assessment; template model.
Published by Oxford University Press 2023.
Conflict of interest statement
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





Similar articles
-
Comparing computational times for simulations when using PBPK model template and stand-alone implementations of PBPK models.Front Toxicol. 2025 Feb 19;7:1518769. doi: 10.3389/ftox.2025.1518769. eCollection 2025. Front Toxicol. 2025. PMID: 40046613 Free PMC article.
-
A Model Template Approach for Rapid Evaluation and Application of Physiologically Based Pharmacokinetic Models for Use in Human Health Risk Assessments: A Case Study on Per- and Polyfluoroalkyl Substances.Toxicol Sci. 2021 Aug 3;182(2):215-228. doi: 10.1093/toxsci/kfab063. Toxicol Sci. 2021. PMID: 34077538 Free PMC article.
-
Translational research to develop a human PBPK models tool kit-volatile organic compounds (VOCs).J Toxicol Environ Health A. 2012;75(1):6-24. doi: 10.1080/15287394.2012.625546. J Toxicol Environ Health A. 2012. PMID: 22047160 Free PMC article.
-
Toxicokinetic modeling and its applications in chemical risk assessment.Toxicol Lett. 2003 Feb 18;138(1-2):9-27. doi: 10.1016/s0378-4274(02)00375-2. Toxicol Lett. 2003. PMID: 12559690 Review.
-
Development of a human Physiologically Based Pharmacokinetic (PBPK) Toolkit for environmental pollutants.Int J Mol Sci. 2011;12(11):7469-80. doi: 10.3390/ijms12117469. Epub 2011 Oct 31. Int J Mol Sci. 2011. PMID: 22174611 Free PMC article. Review.
Cited by
-
Comparing computational times for simulations when using PBPK model template and stand-alone implementations of PBPK models.Front Toxicol. 2025 Feb 19;7:1518769. doi: 10.3389/ftox.2025.1518769. eCollection 2025. Front Toxicol. 2025. PMID: 40046613 Free PMC article.
References
-
- Andersen ME, Clewell HJ III, Gargas ML, Macnaughton MG, Reitz RH, Nolan RJ, and Mckenna MJ (1991). Physiologically based pharmacokinetic modeling with dichloromethane, its metabolite, carbon monoxide, and blood carboxyhemoglobin in rats and humans. Toxicol. Appl. Pharmacol. 108, 14–27. - PubMed
-
- Bois FY (2009). GNU MCSim: Bayesian statistical inference for SBML-coded systems biology models. Bioinformatics 25, 1453–1454. - PubMed
-
- Brightman FA, Leahy DE, Searle GE, and Thomas S. (2006). Application of a generic physiologically based pharmacokinetic model to the estimation of xenobiotic levels in rat plasma. Drug Metab. Dispos. 34, 84–93. - PubMed
-
- Campbell JL Jr, Clewell RA, Gentry PR, Andersen ME, and Clewell HJ III. (2012). Physiologically based pharmacokinetic/toxicokinetic modeling. Methods Mol. Biol. 929, 439–499. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

