Nipah Virus Bangladesh Infection Elicits Organ-Specific Innate and Inflammatory Responses in the Marmoset Model
- PMID: 36869692
- PMCID: PMC10469344
- DOI: 10.1093/infdis/jiad053
Nipah Virus Bangladesh Infection Elicits Organ-Specific Innate and Inflammatory Responses in the Marmoset Model
Abstract
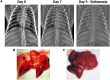
The common marmoset (Callithrix jacchus) is increasingly recognized as an ideal nonhuman primate (NHP) at high biocontainment due to its smaller size and relative ease of handling. Here, we evaluated the susceptibility and pathogenesis of Nipah virus Bangladesh strain (NiVB) infection in marmosets at biosafety level 4. Infection via the intranasal and intratracheal route resulted in fatal disease in all 4 infected marmosets. Three developed pulmonary edema and hemorrhage as well as multifocal hemorrhagic lymphadenopathy, while 1 recapitulated neurologic clinical manifestations and cardiomyopathy on gross pathology. Organ-specific innate and inflammatory responses were characterized by RNA sequencing in 6 different tissues from infected and control marmosets. Notably, a unique transcriptome was revealed in the brainstem of the marmoset exhibiting neurological signs. Our results provide a more comprehensive understanding of NiV pathogenesis in an accessible and novel NHP model, closely reflecting clinical disease as observed in NiV patients.
Keywords: Nipah virus Bangladesh; RNA-seq; cardiomyopathy; common marmoset; inflammatory response; neurological disease; nonhuman primate; pathogenesis; respiratory disease.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



Similar articles
-
A Lethal Aerosol Exposure Model of Nipah Virus Strain Bangladesh in African Green Monkeys.J Infect Dis. 2020 May 11;221(Suppl 4):S431-S435. doi: 10.1093/infdis/jiz469. J Infect Dis. 2020. PMID: 31665351
-
Comparison of the pathogenicity of Nipah virus isolates from Bangladesh and Malaysia in the Syrian hamster.PLoS Negl Trop Dis. 2013;7(1):e2024. doi: 10.1371/journal.pntd.0002024. Epub 2013 Jan 17. PLoS Negl Trop Dis. 2013. PMID: 23342177 Free PMC article.
-
An Intranasal Exposure Model of Lethal Nipah Virus Infection in African Green Monkeys.J Infect Dis. 2020 May 11;221(Suppl 4):S414-S418. doi: 10.1093/infdis/jiz391. J Infect Dis. 2020. PMID: 31665362 Free PMC article.
-
Recent advances in the understanding of Nipah virus immunopathogenesis and anti-viral approaches.F1000Res. 2019 Oct 16;8:F1000 Faculty Rev-1763. doi: 10.12688/f1000research.19975.1. eCollection 2019. F1000Res. 2019. PMID: 31656582 Free PMC article. Review.
-
[Nipah virus infections].Nihon Rinsho. 2005 Dec;63(12):2143-53. Nihon Rinsho. 2005. PMID: 16363687 Review. Japanese.
Cited by
-
A Survey of Henipavirus Tropism-Our Current Understanding from a Species/Organ and Cellular Level.Viruses. 2023 Oct 4;15(10):2048. doi: 10.3390/v15102048. Viruses. 2023. PMID: 37896825 Free PMC article. Review.
-
Henipaviruses: epidemiology, ecology, disease, and the development of vaccines and therapeutics.Clin Microbiol Rev. 2025 Mar 13;38(1):e0012823. doi: 10.1128/cmr.00128-23. Epub 2024 Dec 23. Clin Microbiol Rev. 2025. PMID: 39714175 Free PMC article. Review.
-
Pathogenicity and virulence of henipaviruses.Virulence. 2023 Dec;14(1):2273684. doi: 10.1080/21505594.2023.2273684. Epub 2023 Nov 10. Virulence. 2023. PMID: 37948320 Free PMC article. Review.
-
Animal Models for Henipavirus Research.Viruses. 2023 Sep 22;15(10):1980. doi: 10.3390/v15101980. Viruses. 2023. PMID: 37896758 Free PMC article. Review.
-
Beyond neurology: unravelling Nipah virus's cardiovascular conundrum-an editorial.Ann Med Surg (Lond). 2024 May 8;86(6):3204-3205. doi: 10.1097/MS9.0000000000002149. eCollection 2024 Jun. Ann Med Surg (Lond). 2024. PMID: 38846894 Free PMC article. No abstract available.
References
-
- Looi L-M, Chua K-B. Lessons from the Nipah virus outbreak in Malaysia. Malays J Pathol 2007; 29:63–7. - PubMed
-
- Howley PM, Knipe DM, Whelan SPJ, Fields BN, eds. Fields virology. Vol. 1, Emerging viruses. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2021.
-
- Lee B, Broder CC, Wang L-F. Henipaviruses: Hendra and Nipah viruses. In: Howley PM, Knipe DM, Whelan SPJ, Fields BN eds. Fields virology. Vol. 1 Emerging viruses. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2021:559–95.
-
- Goh KJ, Tan CT, Chew NK, et al. . Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med 2000; 342:1229–35. - PubMed
-
- Hossain MJ, Gurley ES, Montgomery JM, et al. . Clinical presentation of Nipah virus infection in Bangladesh. Clin Infect Dis 2008; 46:977–84. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

