The Defenders of the Alveolus Succumb in COVID-19 Pneumonia to SARS-CoV-2 and Necroptosis, Pyroptosis, and PANoptosis
- PMID: 36869698
- PMCID: PMC10226656
- DOI: 10.1093/infdis/jiad056
The Defenders of the Alveolus Succumb in COVID-19 Pneumonia to SARS-CoV-2 and Necroptosis, Pyroptosis, and PANoptosis
Abstract
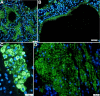
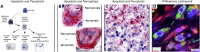
Alveolar type II (ATII) pneumocytes as defenders of the alveolus are critical to repairing lung injury. We investigated the ATII reparative response in coronavirus disease 2019 (COVID-19) pneumonia, because the initial proliferation of ATII cells in this reparative process should provide large numbers of target cells to amplify severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus production and cytopathological effects to compromise lung repair. We show that both infected and uninfected ATII cells succumb to tumor necrosis factor-α (TNF)-induced necroptosis, Bruton tyrosine kinase (BTK)-induced pyroptosis, and a new PANoptotic hybrid form of inflammatory cell death mediated by a PANoptosomal latticework that generates distinctive COVID-19 pathologies in contiguous ATII cells. Identifying TNF and BTK as the initiators of programmed cell death and SARS-CoV-2 cytopathic effects provides a rationale for early antiviral treatment combined with inhibitors of TNF and BTK to preserve ATII cell populations, reduce programmed cell death and associated hyperinflammation, and restore functioning alveoli in COVID-19 pneumonia.
Keywords: BTK; COVID-19 pneumonia; PANoptosis; SARS-CoV-2; TNF; lung repair; necroptosis; pyroptosis; type II pneumocytes.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures





Update of
-
The Defenders of the Alveolus Succumb in COVID-19 Pneumonia to SARS-CoV-2, Necroptosis, Pyroptosis and Panoptosis.bioRxiv [Preprint]. 2022 Aug 8:2022.08.06.503050. doi: 10.1101/2022.08.06.503050. bioRxiv. 2022. Update in: J Infect Dis. 2023 May 29;227(11):1245-1254. doi: 10.1093/infdis/jiad056. PMID: 35982650 Free PMC article. Updated. Preprint.
Similar articles
-
The Defenders of the Alveolus Succumb in COVID-19 Pneumonia to SARS-CoV-2, Necroptosis, Pyroptosis and Panoptosis.bioRxiv [Preprint]. 2022 Aug 8:2022.08.06.503050. doi: 10.1101/2022.08.06.503050. bioRxiv. 2022. Update in: J Infect Dis. 2023 May 29;227(11):1245-1254. doi: 10.1093/infdis/jiad056. PMID: 35982650 Free PMC article. Updated. Preprint.
-
Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes.bioRxiv [Preprint]. 2020 Nov 13:2020.10.29.361048. doi: 10.1101/2020.10.29.361048. bioRxiv. 2020. Update in: Cell. 2021 Jan 7;184(1):149-168.e17. doi: 10.1016/j.cell.2020.11.025. PMID: 33140051 Free PMC article. Updated. Preprint.
-
Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes.Cell. 2021 Jan 7;184(1):149-168.e17. doi: 10.1016/j.cell.2020.11.025. Epub 2020 Nov 19. Cell. 2021. PMID: 33278357 Free PMC article.
-
From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways.Comput Struct Biotechnol J. 2021 Aug 3;19:4641-4657. doi: 10.1016/j.csbj.2021.07.038. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34504660 Free PMC article. Review.
-
Pyroptotic cell death in SARS-CoV-2 infection: revealing its roles during the immunopathogenesis of COVID-19.Int J Biol Sci. 2022 Sep 21;18(15):5827-5848. doi: 10.7150/ijbs.77561. eCollection 2022. Int J Biol Sci. 2022. PMID: 36263178 Free PMC article. Review.
Cited by
-
Differential activation of programmed cell death in patients with severe SARS-CoV-2 infection.Cell Death Discov. 2023 Nov 20;9(1):420. doi: 10.1038/s41420-023-01715-4. Cell Death Discov. 2023. PMID: 37985756 Free PMC article.
-
Role of Lipopolysaccharides in the Inflammation and Pyroptosis of Alveolar Epithelial Cells in Acute Lung Injury and Acute Respiratory Distress Syndrome.J Inflamm Res. 2024 Aug 30;17:5855-5869. doi: 10.2147/JIR.S479051. eCollection 2024. J Inflamm Res. 2024. PMID: 39228678 Free PMC article. Review.
-
Targeting necroptosis: a promising avenue for respiratory disease treatment.Cell Commun Signal. 2024 Aug 28;22(1):418. doi: 10.1186/s12964-024-01804-6. Cell Commun Signal. 2024. PMID: 39192326 Free PMC article. Review.
-
Deciphering the molecular nexus between Omicron infection and acute kidney injury: a bioinformatics approach.Front Mol Biosci. 2024 Jul 4;11:1340611. doi: 10.3389/fmolb.2024.1340611. eCollection 2024. Front Mol Biosci. 2024. PMID: 39027131 Free PMC article.
-
SARS-CoV-2 and its ORF3a, E and M viroporins activate inflammasome in human macrophages and induce of IL-1α in pulmonary epithelial and endothelial cells.Cell Death Discov. 2024 Apr 25;10(1):191. doi: 10.1038/s41420-024-01966-9. Cell Death Discov. 2024. PMID: 38664396 Free PMC article.
References
-
- World Health Organization . World Health Organization coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed 31 January 2023.
-
- Van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. . A guide to immunotherapy for COVID-19. Nat Med 2022; 8:39–50. - PubMed

