The Application of Guanidinium to Improve Biomolecule Quality in Fixed, Paraffin-embedded Tissue
- PMID: 36869703
- PMCID: PMC10088100
- DOI: 10.1369/00221554231159451
The Application of Guanidinium to Improve Biomolecule Quality in Fixed, Paraffin-embedded Tissue
Abstract
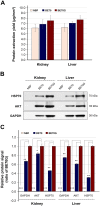
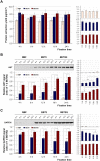
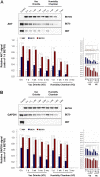
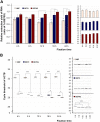
Neutral buffered formalin (NBF) is the most common fixative in clinical applications. However, NBF damages proteins and nucleic acids, limiting the quality of proteomic and nucleic acid-based assays. Prior studies have demonstrated that BE70, a fixative of buffered 70% ethanol, has many benefits over NBF but the degradation of proteins and nucleic acids in archival paraffin blocks remain a challenge. Thus, we evaluated the addition of guanidinium salts to BE70 with the hypothesis that this may protect RNA and protein. Guanidinium salt supplemented BE70 (BE70G)-fixed tissue is comparable with that of BE70 via histology and immunohistochemistry. Western blot analysis also revealed that HSP70, AKT, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression signals in BE70G-fixed tissue were higher than those in BE70-fixed tissue. The quality of nucleic acids extracted from BE70G-fixed, paraffin-embedded tissue was also superior, and BE70G provides improved protein and RNA quality at shorter fixation times than its predecessors. The degradation of proteins, AKT and GAPDH, in archival tissue blocks is also decreased with the addition of guanidinium salt to BE70. In conclusion, BE70G fixative improves the quality of molecular analysis with more rapid fixation of tissue and enhanced long-term storage of paraffin blocks at room temperature for evaluation of protein epitopes.
Keywords: RNA; alcohol; fixation; formaldehyde; guanidinium; histology; immunohistochemistry; protein.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The National Institutes of Health have filed a patent application (WO2017083729A3) for the fixative described herein. Ms. Perry, Dr. Chung, Dr. Star, and Dr. Hewitt are listed as inventors on this patent application; however, the application is assigned to the U.S. Department of Health and Human Services, as the work was performed under official duty (J.-Y.C., R.A.S., and S.M.H.).
Figures


Similar articles
-
Histomorphological and Molecular Assessments of the Fixation Times Comparing Formalin and Ethanol-Based Fixatives.J Histochem Cytochem. 2018 Feb;66(2):121-135. doi: 10.1369/0022155417741467. Epub 2017 Nov 10. J Histochem Cytochem. 2018. PMID: 29125916 Free PMC article.
-
A Buffered Alcohol-Based Fixative for Histomorphologic and Molecular Applications.J Histochem Cytochem. 2016 Jul;64(7):425-40. doi: 10.1369/0022155416649579. Epub 2016 May 24. J Histochem Cytochem. 2016. PMID: 27221702 Free PMC article.
-
Making Formalin-Fixed, Paraffin Embedded Blocks.Methods Mol Biol. 2019;1897:253-268. doi: 10.1007/978-1-4939-8935-5_22. Methods Mol Biol. 2019. PMID: 30539450 Review.
-
Formulation and pH of the Buffered Ethanol Fixative BE70.J Histochem Cytochem. 2017 Apr;65(4):251-252. doi: 10.1369/0022155416687279. J Histochem Cytochem. 2017. PMID: 28347266 Free PMC article. No abstract available.
-
Special symposium: fixation and tissue processing models.Biotech Histochem. 2009 Oct;84(5):185-93. doi: 10.3109/10520290903039052. Biotech Histochem. 2009. PMID: 19886755 Free PMC article. Review.
References
-
- Lewis F, Maughan NJ, Smith V, Hillan K, Quirke P.Unlocking the archive—gene expression in paraffin-embedded tissue. J Pathol. 2001;195(1):66–71. - PubMed
-
- Hewitt SM, Lewis FA, Cao Y, Conrad RC, Cronin M, Danenberg KD, Goralski TJ, Langmore JP, Raja RG, Williams PM, Palma JF, Warrington JA.Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008;132(12):1929–35. - PubMed
-
- Chung JY, Braunschweig T, Hewitt SM.Optimization of recovery of RNA from formalin-fixed, paraffin-embedded tissue. Diagn Mol Pathol. 2006;15(4):229–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

