Diagnosis and Clinical Features of Perianal Lesions in Newly Diagnosed Crohn's Disease: Subgroup Analysis from Inception Cohort Registry Study of Patients with Crohn's Disease (iCREST-CD)
- PMID: 36869815
- PMCID: PMC10441562
- DOI: 10.1093/ecco-jcc/jjad038
Diagnosis and Clinical Features of Perianal Lesions in Newly Diagnosed Crohn's Disease: Subgroup Analysis from Inception Cohort Registry Study of Patients with Crohn's Disease (iCREST-CD)
Abstract
Background and aims: Perianal lesion is a refractory phenotype of Crohn's disease [CD] with significantly diminished quality of life. We evaluated the clinical characteristics of perianal lesions in newly diagnosed CD patients and the impact of perianal lesions on the quality of life in Japanese patients with CD.
Methods: Patients newly diagnosed with CD after June 2016 were included between December 2018 and June 2020 from the Inception Cohort Registry Study of Patients with CD [iCREST-CD].
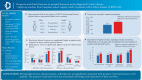
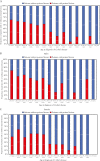
Results: Perianal lesions were present in 324 [48.2%] of 672 patients with newly diagnosed CD; 71.9% [233/324] were male. The prevalence of perianal lesions was higher in patients aged <40 years vs ≥40 years, and it decreased with age. Perianal fistula [59.9%] and abscess [30.6%] were the most common perianal lesions. In multivariate analyses, male sex, age <40 years and ileocolonic disease location were significantly associated with a high prevalence of perianal lesions, whereas stricturing behaviour and alcohol intake were associated with low prevalence. Fatigue was more frequent [33.3% vs 21.6%] while work productivity and activity impairment-work time missed [36.3% vs 29.5%] and activity impairment [51.9% vs 41.1%] were numerically higher in patients with than those without perianal lesions.
Conclusions: At the time of CD diagnosis, approximately half of the patients had perianal lesions; perianal abscesses and perianal fistulas were the most common. Young age, male sex, disease location and behaviour were significantly associated with the presence of perianal lesions. The presence of perianal lesion was associated with fatigue and impairment of daily activities.
Clinical trials registry: University Hospital Medical Information Network Clinical Trials Registry System [UMIN-CTR, UMIN000032237].
Keywords: Crohn’s disease; patient-reported outcomes; perianal lesion.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
Yoko Murata, Shinichi Yoshigoe, Shinya Nagasaka and Tsutomu Yajima are employees of Janssen Pharmaceutical K.K. and may hold stock or stock options of Johnson & Johnson.
Kenji Watanabe reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from EA Pharma, Takeda and EP CRSU and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from JIMRO, EA Pharma, Takeda and Abbvie Japan, and held leadership or fiduciary roles in the Finance Committee of JSIBD, Japan Small Intestine Society, The Japanese Society of Mucosal Immunology and Public Relations Committee of The Japanese Society for Mucosal Immunology.
Tadakazu Hisamatsu reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Janssen Pharmaceutical K.K, Alfresa, EA Pharma, Mitsubishi Tanabe, AbbVie GK, JIMRO, Zeria, Daiichi-Sankyo,Kyorin. Nippon Kayaku, Takeda, Pfizer Inc. and Mochida; consulting fees for advisory boards from Janssen Pharmaceutical K.K, AbbVie GK, Takeda, Pfizer Inc. and Gilead; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen Pharmaceutical K.K, EA Pharma, Mitsubishi Tanabe, AbbVie GK, JIMRO, Kyorin, Takeda, Pfizer Inc., Mochida and Gilead; and held leadership or fiduciary roles in MHLW, Grant-in-Aid for Scientific Research on Intractable Diseases, ‘Research on Intractable Inflammatory Bowel Disorders’, JSIBD, Japan Small Intestine Society, The Japanese Society for Mucosal Immunology, and The Japanese Society of Clinical Immunology. Ryuichi Okamoto reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Mitsubishi Tanabe, Zeria, Mochida and Takeda; and held leadership or fiduciary roles in the Research Grant Committee of JSIBD and International Exchange Committee of JSIBD.
Katsuyoshi Matsuoka reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from AbbVie, EA Pharma, Mitsubishi Tanabe Pharma, Mochida, Kyorin, Kissei, JIMRO and Nippon Kayaku; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie, EA Pharma, Mochida, Kyorin, Kissei, Janssen, JIMRO, Gilead Sciences, Pfizer, Takeda, Zeria, Miyarisan, Nippon Kayaku, Celltrion Healthcare, Eli Lilly and Mitsubishi Tanabe Pharma; and held leadership or fiduciary roles in the Clinical Epidemiology Committee of JSIBD, The Japanese Society for Mucosal Immunology, International Exchange Committee of The Japanese Society for Mucosal Immunology, AOCC Governing Board, Japan Small Intestine Society, and MHLW, Grant-in-Aid for Scientific Research on Intractable Diseases, ‘Research on Intractable Inflammatory Bowel Disorders’.
Shigeki Bamba reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from AbbVie GK; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie GK, Mitsubishi Tanabe, Kyorin, Janssen, Mochida and EA Pharma; and held leadership or fiduciary roles in the Japan Small Intestine Committee, The Japanese Society for Mucosal Immunology The Conflict of Interest and Ethics Committee of The Japanese Society for Mucosal Immunology.
Toshimitsu Fujii reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Janssen Pharmaceutical K.K, AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, EA Pharma, Kissei, Takeda, Alfresa Corporation, Celgene, Eli Lilly, Gilead Sciences and Sekisui Medical; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen Pharmaceutical K.K, AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, EA Pharma, Kissei, Kyorin, Mitsubishi Tanabe, Mochida, Nichiiko, Nippon Kayaku, Takeda and Zeria; and held leadership or fiduciary roles in the Clinical Epidemiology Committee of JSIBD and Specialist system Committee of JSIBD.
Reiko Kunisaki reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Janssen Pharmaceutical K.K, AbbVie GK, EA Pharma, Kissei, Mitsubishi Tanabe, Takeda, Zeria, Ajinomoto, Eli Lilly and Pfizer Inc.; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen Pharmaceutical K.K, AbbVie GK, EA Pharma, Kissei, Kyorin, Nippon Kayaku, Mitsubishi Tanabe, Takeda and Zeria; and held leadership or fiduciary roles in the Committee on Public Information of JSIBD.
Koichiro Matsuda reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received payment or honoraria [received by his Institution] for lectures, presentations, speakers bureaus, manuscript writing or educational events from Jansen Pharmaceutical K.K, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co.,Ltd., AbbVie Inc. and Eli Lilly Japan K.K.; participated in the Data Safety Monitoring Board or Advisory Board of Jansen Pharmaceutical K.K.; and holds stock or stock options of Gilead Sciences, Inc.
Minoru Matsuura reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received payment or honoraria [received by his Institution] for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Co., AbbVie GK, Takeda Pharmaceutical Co., Ltd, Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd, Viatris Inc., Sandoz AG, Nippon Kayaku Co., Ltd, JIMRO Co., Ltd, EA Pharma Co., Ltd and ZERIA Pharmaceutical Co., Ltd; and held leadership or fiduciary roles in the Clinical epidemiology committee of JSIBD, The Japanese Society for Mucosal Immunology, Committee on Public Information of the Japanese Society for Mucosal Immunology, and Japan Small Intestine Society.
Yohei Mikami reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grant from Janssen Pharmaceutical K.K., and held leadership or fiduciary roles in the Clinical Epidemiology Committee of JSIBD, Research Grant Committee of JSIBD, and The Japanese Society for Mucosal Immunology. Satoshi Motoya reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from AbbVie GK, Takeda, Janssen, Pfizer Japan Inc. and EA Pharma; payment or honoraria [received by his Institution] for lectures, presentations, speakers bureaus, manuscript writing or educational events from Mitsubishi Tanabe, AbbVie GK, Mochida, Takeda and Janssen; held leadership or fiduciary roles in the Public Relations Committee of JSIBD, Clinical Epidemiology Committee of JSIBD and Japan Small Intestine Society.
Naoki Ohmiya reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Abbvie GK, Daiichi Sankyo, Bristol-Myers Squibb, Takeda, Eli Lilly, Mitsubishi Tanabe and Nippon Kayaku; payment or honoraria [received by his Institution] for lectures, presentations, speakers bureaus, manuscript writing or educational events from EA Pharma and Janssen; and held leadership or fiduciary roles in the Japan Small Intestine Society.
Hisashi Shiga reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Janssen Pharmaceutical K.K., payment or honoraria [received by his Institution] for lectures, presentations, speakers bureaus, manuscript writing or educational events from Mitsubishi Tanabe Pharma, EA Pharma Co. Ltd, Takeda Pharmaceutical Co. Ltd, AbbVie Inc., Janssen Pharmaceutical K.K. and Pfizer Inc.; and held leadership or fiduciary roles in the Education Committee of JSIBD and Specialist System Committee of JSIBD. Takahiro Shimoyama reports no conflict of interest for the submitted manuscript and outside this publication during the past 36 months.
Shinichiro Shinzaki reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Sekisui Medical; consulting fees from Abbvie Inc., Janssen Pharmaceutical K.K., Takeda Pharmaceutical; payment or honoraria [received by his Institution] for lectures, presentations, speakers bureaus, manuscript writing or educational events from Alfresa Pharma Corporation, Astra Zeneka K.K., EA Pharma, Eisai, Gilead Sciences, Kissei, Kyorin, Janssen Pharmaceutical K.K., JIMRO, Mitsubishi-Tanabe Pharma, Mochida, Nippon Kayaku, Pfizer, Sekisui Medical, Takeda and Zeria; and held leadership or fiduciary roles in the International Exchange Committee of JSIBD, Clinical Epidemiology Committee of JSIBD, The Japanese Society of Gastroenterological Immunology, Board of education of The Japanese Society for Mucosal Immunology. Noritaka Takatsu reports no conflict of interest for the submitted manuscript and outside this publication during the past 36 months.
Takehiro Torisu reports no conflict of interest for the submitted manuscript and outside this publication during the past 36 months. Takayuki Yamamoto reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Janssen Pharmaceutical K.K., and held leadership or fiduciary roles in the International exchange committee of JSIBD and Clinical Epidemiology Committee of JSIBD.
Taku Kobayashi reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants [paid to his institution] from Abbie GK, Activaid, Alfresa Pharma Corporation, JMDC Inc., Gilead Sciences, Inc., Nippon Kayaku Co., Ltd, Eli Lilly Japan K.K., Mochida Pharmaceutical Co., Ltd, Janssen Pharmaceutical K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd, Bristol-Myers Squibb, Mitsubishi Tanabe Pharma, Zeria Nippon Kayaku Co., Ltd, Otsuka Holdings, EA Pharma, JIMRO, Kyorin and Google Asia Pacific Pte.Ltd; payment or honoraria [received by his Institution] for lectures, presentations, speakers bureaus, manuscript writing or educational events from Takeda Pharmaceutical Co., Ltd., Activaid, Alfresa Pharma Corporation, Zeria, Kyorin, Nippon Kayaku Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Abbie GK, Pfizer Japan Inc., Janssen Pharmaceutical K.K., Thermo Fisher Diagnostics K.K., JIMRO and Galapagos; payment for expert testimony from Janssen Pharmaceutical K.K., EA Pharma Co., Ltd, KISSEI Pharmaceutical Co., Ltd, Takeda Pharmaceutical Co., Ltd, Activaid, Pfizer Japan Inc., Nippon Kayaku Co., Ltd, Alfresa Pharma Corporation, Kyorin Pharmaceutical Co., Ltd, Abbie GK, Mochida Pharmaceutical Co., Ltd and Mitsubishi Tanabe Pharma Corporation; and held leadership or fiduciary roles in the MHLW, Grant-in-Aid for Scientific Research on Intractable Diseases, ‘Research on Intractable Inflammatory Bowel Disorders’, International Exchange Committee of JSIBD, The Japanese Society for Mucosal Immunology, International Exchange Committee of the Japanese Society for Mucosal Immunology. Atsuo Maemoto reports no conflict of interest for the submitted manuscript and outside this publication during the past 36 months.
Takayuki Matsumoto reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Janssen Pharmaceutical K.K. and Mitsubishi Tanabe; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen Pharmaceutical K.K., Tanabe Mitsubishi Pharmaceutical, EA Pharma, Abbvie and Takeda Pharmaceutical; held leadership or fiduciary roles in the MHLW, Grant-in-Aid for Scientific Research on Intractable Diseases, ‘Research on Intractable Inflammatory Bowel Disorders’, JSIBD, General Affairs Committee of JSIBD, Japan Small Intestine Society, The Japanese Gastroenterological Association and Japanese Gastroenterological Endoscopy Society.
Hiroshi Nakase reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Hoya Group Pentax Medical, Boehringer Ingelheim GmbH and Bristol-Myers Squibb; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen Pharmaceutical K.K.; and held leadership or fiduciary roles in the MHLW, Grant-in-Aid for Scientific Research on Intractable Diseases, ‘Research on Intractable Inflammatory Bowel Disorders’, JSIBD, JSIBD, International Exchange Committee, Bulletin editorial committee of JSIBD, Japan Small Intestine Society, The Japanese Society for Mucosal Immunology, Finance committee of the Japanese Society for Mucosal Immunology, Society Steering Committee of the Japanese Society for Mucosal Immunology, Board of Academic and Board Certified Physicians of The Japanese Society of Clinical Immunology, Certified Physician/Guideline Subcommittee of The Japanese Society of Clinical Immunology, and The Japanese Society of Gastroenterology.
Masayuki Saruta reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants from Mochida Pharmaceutical Co., Ltd, Zeria Pharma Co., Ltd, EA Pharma Co., Ltd, Kissei Pharmaceutical Co., Ltd and EPS Corporation; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbvie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Co., Ltd, Takeda Pharmaceutical Co., Ltd, EA Pharma Co., Ltd and Gilead Sciences K.K., held leadership or fiduciary roles in the MHLW, Grant-in-Aid for Scientific Research on Intractable Diseases, ‘Research on Intractable Inflammatory Bowel Disorders’, General Affairs Committee of JSIBD, AOCC, Japan Small Intestine Society, The Japanese Society for Mucosal Immunology, and Officer Selection Committee of The Japanese Society for Mucosal Immunology.
Akihiro Yamada reports no conflict of interest for the submitted manuscript. Outside this publication during the past 36 months, he has received research grants [paid to institution] from Abbie GK, Mitsubishi Tanabe, EA Pharma, and Mochida.
Figures


References
-
- de Groof EJ, Sahami S, Lucas C, Ponsioen CY, Bemelman WA, Buskens CJ.. Treatment of perianal fistula in Crohn’s disease: a systematic review and meta-analysis comparing seton drainage and anti-tumour necrosis factor treatment. Colorectal Dis 2016;18:667–75. - PubMed
-
- Schwartz DA, Loftus EV Jr, Tremaine WJ, et al. . The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 2002;122:875–80. - PubMed
-
- Kotze PG, Shen B, Lightner A, et al. . Modern management of perianal fistulas in Crohn’s disease: future directions. Gut 2018;67:1181–94. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

