Immunity of Heterologously and Homologously Boosted or Convalescent Individuals Against Omicron BA.1, BA.2, and BA.4/5 Variants
- PMID: 36869832
- PMCID: PMC10345468
- DOI: 10.1093/infdis/jiad057
Immunity of Heterologously and Homologously Boosted or Convalescent Individuals Against Omicron BA.1, BA.2, and BA.4/5 Variants
Abstract
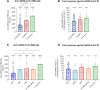
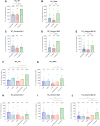
Background: The emerged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variants BA.1, BA.2, and BA.4/5 demonstrate higher transmission and infection rates than previous variants of concern. To evaluate effectiveness of heterologous and homologous booster vaccination, we directly compared cellular and humoral immune responses as well as neutralizing capacity against replication-competent SARS-CoV-2 wild type, Delta, and Omicron variants BA.1, BA.2, and BA.4/5.
Methods: Peripheral blood mononuclear cells and serum samples from 137 participants were investigated, in 3 major groups. Individuals in the first group were vaccinated twice with ChAdOx1 and boosted with a messenger RNA (mRNA) vaccine (BNT162b2 or mRNA-1273); the second group included triple mRNA--vaccinated participants, and the third group, twice-vaccinated and convalescent individuals.
Results: Vaccination and convalescence resulted in the highest SARS-CoV-2-specific antibody levels, stronger T-cell responses, and best neutralization against wild type, Delta Omicron BA.2, and BA.4/5, while a combination of ChAdOx1 and BNT162b2 vaccination elevated neutralizing capacity against Omicron BA.1. In addition, heterologous booster regimens, compared with homologous regimens, showed higher efficacy against Omicron BA.2 as well as BA.4/5.
Conclusions: We showed that twice-vaccinated and convalescent individuals demonstrated the strongest immunity against Omicron BA.2 and BA.4/5 variant, followed by those receiving heterologous and homologous booster vaccine regimens.
Keywords: COVID-19; neutralizing antibodies and T cells; omicron subvariants; vaccines; virus neutralization.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Callaway E. Heavily mutated omicron variant puts scientists on alert. Nature 2021; 600:21. - PubMed
-
- Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature 2022; 602:671–5. - PubMed
-
- Hodcroft E [. Accessed 5 September 2022.]. https://covariants.org/per-variant?variant=22A+%28Omicron%29&variant=22B... . Overview of variants/mutations.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

