Cost-effectiveness of Sodium-Glucose Cotransporter-2 Inhibitors for the Treatment of Heart Failure With Preserved Ejection Fraction
- PMID: 36870047
- PMCID: PMC9985815
- DOI: 10.1001/jamacardio.2023.0077
Cost-effectiveness of Sodium-Glucose Cotransporter-2 Inhibitors for the Treatment of Heart Failure With Preserved Ejection Fraction
Abstract
Importance: Adding a sodium-glucose cotransporter-2 inhibitor (SGLT2-I) to standard-of-care treatment in patients with heart failure with preserved ejection fraction (HFpEF) reduces the risk of a composite outcome of worsening heart failure or cardiovascular mortality, but the cost-effectiveness in US patients with HFpEF is uncertain.
Objective: To evaluate the lifetime cost-effectiveness of standard therapy plus an SGLT2-I compared with standard therapy in individuals with HFpEF.
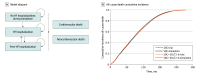
Design, setting, and participants: In this economic evaluation conducted from September 8, 2021, to December 12, 2022, a state-transition Markov model simulated monthly health outcomes and direct medical costs. Input parameters including hospitalization rates, mortality rates, costs, and utilities were extracted from HFpEF trials, published literature, and publicly available data sets. The base-case annual cost of SGLT2-I was $4506. A simulated cohort with similar characteristics as participants of the Empagliflozin in Heart Failure With a Preserved Ejection Fraction (EMPEROR-Preserved) and Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Fraction (DELIVER) trials was used.
Exposures: Standard of care plus SGLT2-I vs standard of care.
Main outcomes and measures: The model simulated hospitalizations, urgent care visits, and cardiovascular and noncardiovascular death. Future medical costs and benefits were discounted by 3% per year. Main outcomes were quality-adjusted life-years (QALYs), direct medical costs (2022 US dollars), and incremental cost-effectiveness ratio (ICER) of SGLT2-I therapy from a US health care sector perspective. The ICER of SGLT2-I therapy was evaluated according to the American College of Cardiology/American Heart Association value framework (high value: <$50 000; intermediate value: $50 000 to <$150 000; and low value: ≥$150 000).
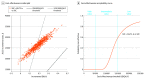
Results: The simulated cohort had a mean (SD) age of 71.7 (9.5) years and 6828 of 12 251 participants (55.7%) were male. Standard of care plus SGLT2-I increased quality-adjusted survival by 0.19 QALYs at an increased cost of $26 300 compared with standard of care. The resulting ICER was $141 200 per QALY gained, with 59.1% of 1000 probabilistic iterations indicating intermediate value and 40.9% indicating low value. The ICER was most sensitive to SGLT2-I costs and effect of SGLT2-I therapy on cardiovascular death (eg, increasing to $373 400 per QALY gained if SGLT2-I therapy was assumed to have no effect on mortality).
Conclusions and relevance: Results of this economic evaluation suggest that at 2022 drug prices, adding an SGLT2-I to standard of care was of intermediate or low economic value compared with standard of care in US adults with HFpEF. Efforts to expand access to SGLT2-I for individuals with HFpEF should be coupled with efforts to lower the cost of SGLT2-I therapy.
Conflict of interest statement
Figures

Comment in
-
Cost-effectiveness of Sodium-Glucose Cotransporter-2 Inhibitors for Patients With Heart Failure and Preserved Ejection Fraction-Living on the Edge.JAMA Cardiol. 2023 May 1;8(5):415-416. doi: 10.1001/jamacardio.2023.0087. JAMA Cardiol. 2023. PMID: 36870043 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

