Neutrophil extracellular traps as a unique target in the treatment of chemotherapy-induced peripheral neuropathy
- PMID: 36870200
- PMCID: PMC10009451
- DOI: 10.1016/j.ebiom.2023.104499
Neutrophil extracellular traps as a unique target in the treatment of chemotherapy-induced peripheral neuropathy
Abstract
Background: Chemotherapy-induced peripheral neuropathy (CIPN) is a severe dose-limiting side effect of chemotherapy and remains a huge clinical challenge. Here, we explore the role of microcirculation hypoxia induced by neutrophil extracellular traps (NETs) in the development of CIPN and look for potential treatment.
Methods: The expression of NETs in plasma and dorsal root ganglion (DRG) are examined by ELISA, IHC, IF and Western blotting. IVIS Spectrum imaging and Laser Doppler Flow Metry are applied to explore the microcirculation hypoxia induced by NETs in the development of CIPN. Stroke Homing peptide (SHp)-guided deoxyribonuclease 1 (DNase1) is used to degrade NETs.
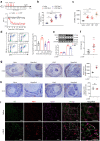
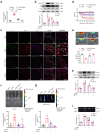
Findings: The level of NETs in patients received chemotherapy increases significantly. And NETs accumulate in the DRG and limbs in CIPN mice. It leads to disturbed microcirculation and ischemic status in limbs and sciatic nerves treated with oxaliplatin (L-OHP). Furthermore, targeting NETs with DNase1 significantly reduces the chemotherapy-induced mechanical hyperalgesia. The pharmacological or genetic inhibition on myeloperoxidase (MPO) or peptidyl arginine deiminase-4 (PAD4) dramatically improves microcirculation disturbance caused by L-OHP and prevents the development of CIPN in mice.
Interpretation: In addition to uncovering the role of NETs as a key element in the development of CIPN, our finding provides a potential therapeutic strategy that targeted degradation of NETs by SHp-guided DNase1 could be an effective treatment for CIPN.
Funding: This study was funded by the National Natural Science Foundation of China81870870, 81971047, 81773798, 82271252; Natural Science Foundation of Jiangsu ProvinceBK20191253; Major Project of "Science and Technology Innovation Fund" of Nanjing Medical University2017NJMUCX004; Key R&D Program (Social Development) Project of Jiangsu ProvinceBE2019732; Nanjing Special Fund for Health Science and Technology DevelopmentYKK19170.
Keywords: Chemotherapy-induced peripheral neuropathy; Microcirculatory disturbance; Neutrophil extracellular traps; SHp-DNase1.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no competing interests.
Figures






References
-
- Park S.B., Goldstein D., Krishnan A.V., et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63:419–437. - PubMed
-
- Seretny M., Currie G.L., Sena E.S., et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461–2470. - PubMed
-
- Wolf S., Barton D., Kottschade L., Grothey A., Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. - PubMed
-
- LoMonaco M., Milone M., Batocchi A.P., Padua L., Restuccia D., Tonali P. Cisplatin neuropathy: clinical course and neurophysiological findings. J Neurol. 1992;239:199–204. - PubMed
-
- Lechner D., Weltermann A. Chemotherapy-induced thrombosis: a role for microparticles and tissue factor? Semin Thromb Hemost. 2008;34:199–203. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

