SARS-CoV-2 immune complex triggers human monocyte necroptosis
- PMID: 36870284
- PMCID: PMC9968621
- DOI: 10.1016/j.intimp.2023.109954
SARS-CoV-2 immune complex triggers human monocyte necroptosis
Abstract
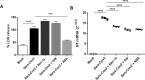
We analyzed the ability of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) itself and SARS-CoV-2-IgG immune complexes to trigger human monocyte necroptosis. SARS-CoV-2 was able to induce monocyte necroptosis dependently of MLKL activation. Necroptosis-associated proteins (RIPK1, RIPK3 and MLKL) were involved in SARS-CoV-2N1 gene expression in monocytes. SARS-CoV-2 immune complexes promoted monocyte necroptosis in a RIPK3- and MLKL-dependent manner, and Syk tyrosine kinase was necessary for SARS-CoV-2 immune complex-induced monocyte necroptosis, indicating the involvement of Fcγ receptors on necroptosis. Finally, we provide evidence that elevated LDH levels as a marker of lytic cell death are associated with COVID-19 pathogenesis.
Keywords: COVD-19 pathogenesis; Fcγ receptors; MLKL; Monocyte; Necroptosis; SARS-CoV-2.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180(7):934–943. - PMC - PubMed
-
- Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.K.S., Schreiner P., Neale G., Vogel P., Webby R., Jonsson C.B., Kanneganti T.D. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184(1):149–168 e17. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

