Activation of neuronal NADPH oxidase NOX2 promotes inflammatory neurodegeneration
- PMID: 36870375
- PMCID: PMC10164140
- DOI: 10.1016/j.freeradbiomed.2023.03.001
Activation of neuronal NADPH oxidase NOX2 promotes inflammatory neurodegeneration
Abstract
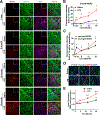
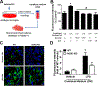
Strong evidence indicates critical roles of NADPH oxidase (a key superoxide-producing enzyme complex during inflammation) in activated microglia for mediating neuroinflammation and neurodegeneration. However, little is known about roles of neuronal NADPH oxidase in neurodegenerative diseases. This study aimed to investigate expression patterns, regulatory mechanisms and pathological roles of neuronal NADPH oxidase in inflammation-associated neurodegeneration. The results showed persistent upregulation of NOX2 (gp91phox; the catalytic subunit of NADPH oxidase) in both microglia and neurons in a chronic mouse model of Parkinson's disease (PD) with intraperitoneal LPS injection and LPS-treated midbrain neuron-glia cultures (a cellular model of PD). Notably, NOX2 was found for the first time to exhibit a progressive and persistent upregulation in neurons during chronic neuroinflammation. While primary neurons and N27 neuronal cells displayed basal expression of NOX1, NOX2 and NOX4, significant upregulation only occurred in NOX2 but not NOX1 or NOX4 under inflammatory conditions. Persistent NOX2 upregulation was associated with functional outcomes of oxidative stress including increased ROS production and lipid peroxidation. Neuronal NOX2 activation displayed membrane translocation of cytosolic p47phox subunit and was inhibited by apocynin and diphenyleneiodonium chloride (two widely-used NADPH oxidase inhibitors). Importantly, neuronal ROS production, mitochondrial dysfunction and degeneration induced by inflammatory mediators in microglia-derived conditional medium were blocked by pharmacological inhibition of neuronal NOX2. Furthermore, specific deletion of neuronal NOX2 prevented LPS-elicited dopaminergic neurodegeneration in neuron-microglia co-cultures separately grown in the transwell system. The attenuation of inflammation-elicited upregulation of NOX2 in neuron-enriched and neuron-glia cultures by ROS scavenger N-acetylcysteine indicated a positive feedback mechanism between excessive ROS production and NOX2 upregulation. Collectively, our findings uncovered crucial contribution of neuronal NOX2 upregulation and activation to chronic neuroinflammation and inflammation-related neurodegeneration. This study reinforced the importance of developing NADPH oxidase-targeting therapeutics for neurodegenerative diseases.
Keywords: Microglia; Mitochondrial dysfunction; NOX2; Neurodegeneration; Neuronal NADPH oxidase; Oxidative stress.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest We state ‘Declarations of interest: none’ in the title page and have a Section “Competing interests” and statement “The authors declare that they have no competing interests” in the manuscript.
Figures





References
-
- Block ML, Zecca L, Hong JS, Microglia-mediated neurotoxicity: uncovering the molecular mechanisms, Nat Rev Neurosci 8(1) (2007) 57–69. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

