Excess Growth Hormone Triggers Inflammation-Associated Arthropathy, Subchondral Bone Loss, and Arthralgia
- PMID: 36870529
- PMCID: PMC10284029
- DOI: 10.1016/j.ajpath.2023.02.010
Excess Growth Hormone Triggers Inflammation-Associated Arthropathy, Subchondral Bone Loss, and Arthralgia
Abstract
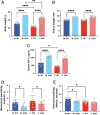
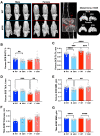
Growth hormone (GH) is a key mediator of skeletal growth. In humans, excess GH secretion due to pituitary adenoma, seen in patients with acromegaly, results in severe arthropathies. This study investigated the effects of long-term excess GH on the knee joint tissues. One year-old wild-type (WT) and bovine GH (bGH) transgenic mice were used as a model for excess GH. bGH mice showed increased sensitivity to mechanical and thermal stimuli, compared with WT mice. Micro-computed tomography analyses of the distal femur subchondral bone revealed significant reductions in trabecular thickness and significantly reduced bone mineral density of the tibial subchondral bone-plate associated with increased osteoclast activity in both male and female bGH compared with WT mice. bGH mice showed severe loss of matrix from the articular cartilage, osteophytosis, synovitis, and ectopic chondrogenesis. Articular cartilage loss in the bGH mice was associated with elevated markers of inflammation and chondrocyte hypertrophy. Finally, hyperplasia of synovial cells was associated with increased expression of Ki-67 and diminished p53 levels in the synovium of bGH mice. Unlike the low-grade inflammation seen in primary osteoarthritis, arthropathy caused by excess GH affects all joint tissues and triggers severe inflammatory response. Data from this study suggest that treatment of acromegalic arthropathy should involve inhibition of ectopic chondrogenesis and chondrocyte hypertrophy.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Claessen K.M., Mazziotti G., Biermasz N.R., Giustina A. Bone and joint disorders in acromegaly. Neuroendocrinology. 2016;103:86–95. - PubMed
-
- Wassenaar M.J., Biermasz N.R., Bijsterbosch J., Pereira A.M., Meulenbelt I., Smit J.W., Roelfsema F., Kroon H.M., Romijn J.A., Kloppenburg M. Arthropathy in long-term cured acromegaly is characterised by osteophytes without joint space narrowing: a comparison with generalised osteoarthritis. Ann Rheum Dis. 2011;70:320–325. - PubMed
-
- Colao A., Marzullo P., Vallone G., Marino V., Annecchino M., Ferone D., De Brasi D., Scarpa R., Oriente P., Lombardi G. Reversibility of joint thickening in acromegalic patients: an ultrasonography study. J Clin Endocrinol Metab. 1998;83:2121–2125. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

