Raltitrexed enhanced antitumor effect of anlotinib in human esophageal squamous carcinoma cells on proliferation, invasiveness, and apoptosis
- PMID: 36870981
- PMCID: PMC9985835
- DOI: 10.1186/s12885-023-10691-y
Raltitrexed enhanced antitumor effect of anlotinib in human esophageal squamous carcinoma cells on proliferation, invasiveness, and apoptosis
Abstract
Background: Anlotinib is a multi-targeted receptor tyrosine kinase inhibitor (TKI) which has exhibited encouraging clinical activity in advanced non-small cell lung cancer (NSCLC) and soft tissue sarcoma. Raltitrexed is well known to be effective in the treatment of colorectal cancer in China. The present study aims to investigate the combinatory antitumor effect of anlotinib and raltitrexed on human esophageal squamous carcinoma cells and further explore the molecular mechanisms in vitro.
Methods: Human esophageal squamous cell lines KYSE-30 and TE-1 were treated with anlotinib or raltitrexed, or both, then cell proliferation was measured by MTS and colony formation assay; cell migration and invasion were detected by wound-healing and transwell assays; cell apoptosis rate was studied by flow cytometry and the transcription of apoptosis-associated proteins were monitored by quantitative polymerase chain reaction (qPCR) analysis. Finally, western blot was performed to check phosphorylation of apoptotic proteins after treatment.
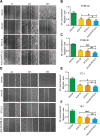
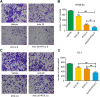
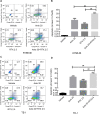
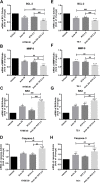
Results: Treatment with raltitrexed and anlotinib showed enhanced inhibitory effects on cell proliferation, migration and invasiveness compared with raltitrexed or anlotinib monotherapy. Meanwhile, raltitrexed combined with anlotinib strongly increased cell apoptosis percentage. Moreover, the combined treatment down-regulated mRNA level of the anti-apoptotic protein Bcl-2 and invasiveness-associated protein matrix metalloproteinases-9 (MMP-9), while up-regulated pro-apoptotic Bax and caspase-3 transcription. Western blotting showed that the combination of raltitrexed and anlotinib could inhibit the expression of phosphorylated Akt (p-Akt), Erk (p-Erk) and MMP-9.
Conclusions: This study indicated that raltitrexed enhanced the antitumor effects of anlotinib on human ESCC cells by down-regulating phosphorylation of Akt and Erk, providing a novel treatment option for patients with esophageal squamous cell carcinoma (ESCC).
Keywords: Anlotinib; Antitumor; ESCC; P-Akt; P-Erk; Raltitrexed.
© 2023. The Author(s).
Conflict of interest statement
All the authors declared that they had no competing interests.
Figures


Similar articles
-
Raltitrexed Enhances the Antitumor Effect of Apatinib in Human Esophageal Squamous Carcinoma Cells via Akt and Erk Pathways.Onco Targets Ther. 2020 Dec 1;13:12325-12339. doi: 10.2147/OTT.S276125. eCollection 2020. Onco Targets Ther. 2020. PMID: 33293826 Free PMC article.
-
[Aumolertinib combined with anlotinib inhibits proliferation of non-small cell lung cancer cells by down-regulating the PI3K/AKT pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Oct 20;44(10):1965-1975. doi: 10.12122/j.issn.1673-4254.2024.10.15. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39523097 Free PMC article. Chinese.
-
Anlotinib Overcomes Multiple Drug Resistant Colorectal Cancer Cells via Inactivating PI3K/AKT Pathway.Anticancer Agents Med Chem. 2021;21(15):1987-1995. doi: 10.2174/1871520621666210112113852. Anticancer Agents Med Chem. 2021. PMID: 33438566
-
Research Progress on Mechanism and Management of Adverse Drug Reactions of Anlotinib.Drug Des Devel Ther. 2023 Nov 15;17:3429-3437. doi: 10.2147/DDDT.S426898. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 38024530 Free PMC article. Review.
-
Anlotinib as a molecular targeted therapy for tumors.Oncol Lett. 2020 Aug;20(2):1001-1014. doi: 10.3892/ol.2020.11685. Epub 2020 May 28. Oncol Lett. 2020. PMID: 32724339 Free PMC article. Review.
Cited by
-
Effect of cervical paraesophageal lymph node metastasis versus supraclavicular lymph node metastasis on the overall survival of patients with thoracic esophageal squamous cell carcinoma: an observational study.Ann Med Surg (Lond). 2024 Mar 18;86(5):2518-2523. doi: 10.1097/MS9.0000000000001955. eCollection 2024 May. Ann Med Surg (Lond). 2024. PMID: 38694352 Free PMC article.
-
Repurposing of Raltitrexed as an Effective G9a/EHMT2 Inhibitor and Promising Anti-Alzheimer's Agent.ACS Med Chem Lett. 2023 Oct 12;14(11):1531-1536. doi: 10.1021/acsmedchemlett.3c00344. eCollection 2023 Nov 9. ACS Med Chem Lett. 2023. PMID: 37974951 Free PMC article.
-
BMS-794833 reduces anlotinib resistance in osteosarcoma by targeting the VEGFR/Ras/CDK2 pathway.J Bone Oncol. 2024 Mar 16;45:100594. doi: 10.1016/j.jbo.2024.100594. eCollection 2024 Apr. J Bone Oncol. 2024. PMID: 38532893 Free PMC article.
References
-
- Sardaro A, Ferrari C, Carbonara R, Altini C, Lavelli V, Rubini G. Synergism between immunotherapy and radiotherapy in esophageal Cancer: an overview of current knowledge and future perspectives. Cancer Biother Radiopharm. 2021;36(2):123–132. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

