Effect of low-dose aspirin on urinary 11-dehydro-thromboxane B2 in the ASCEND (A Study of Cardiovascular Events iN Diabetes) randomized controlled trial
- PMID: 36871000
- PMCID: PMC9985834
- DOI: 10.1186/s13063-023-07198-z
Effect of low-dose aspirin on urinary 11-dehydro-thromboxane B2 in the ASCEND (A Study of Cardiovascular Events iN Diabetes) randomized controlled trial
Abstract
Background: Aspirin is widely used for cardioprotection with its antiplatelet effects due to the blocking of thromboxane A2 production. However, it has been suggested that platelet abnormalities in those with diabetes prevent adequate suppression with once daily aspirin.
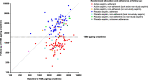
Methods: In the ASCEND randomized double-blind trial of aspirin 100 mg once daily versus placebo in participants with diabetes but no history of cardiovascular disease, suppression was assessed by measuring 11-dehydro-thromboxane B2 excretion in urine (U-TXM) in a randomly selected sample of 152 participants (76 aspirin arm, 74 placebo arm), plus 198 (93 aspirin arm, 105 placebo arm) adherent to study drugs and selected to maximize the numbers ingesting their last tablet 12-24 h before urine sampling. U-TXM was assayed using a competitive ELISA assay in samples mailed a mean of 2 years after randomization, with time since taking last aspirin/placebo tablet recorded at the time of sample provision. Effective suppression (U-TXM < 1500 pg/mg creatinine) and percentage reductions in U-TXM by aspirin allocation were compared.
Results: In the random sample, U-TXM was 71% (95% CI 64-76%) lower among aspirin vs placebo-allocated participants. Among adherent participants in the aspirin arm, U-TXM was 72% (95% CI 69-75%) lower than in the placebo arm and 77% achieved effective suppression overall. Suppression was similar among those who ingested their last tablet more than 12 h before urine sampling with levels in the aspirin arm 72% (95% CI 67-77%) lower than in the placebo arm and 70% achieving effective suppression.
Conclusions: Daily aspirin significantly reduces U-TXM in participants with diabetes, including at 12-24 h after ingestion.
Trial registration: ISRCTN ISRCTN60635500. Registered on 1 Sept 2005; ClinicalTrials.gov NCT00135226. Registered on 24 Aug 2005.
Keywords: 11-Dehydro-thromboxane B2; Daily low-dose aspirin; Diabetes; Randomized placebo-controlled trial.
© 2023. The Author(s).
Conflict of interest statement
SP, MM, GB, SC, MRH, RC, LB, and JA work in the Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU) of the Nuffield Department of Population Health at the University of Oxford. The Clinical Trial Service Unit & Epidemiological Studies Unit has a staff policy of not taking any personal payments directly or indirectly from industry (with reimbursement sought only for the costs of travel and accommodation to attend scientific meetings). CTSU has received research grants from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, The Medicines Company, Merck, Mylan, Novartis, Pfizer, Roche, Schering, and Solvay, which are governed by University of Oxford contracts that protect the researchers’ independence. SP and RC are co-inventors of a genetic test for statin-related myopathy risk but receive no income from it.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CH/1996001/9454/BHF_/British Heart Foundation/United Kingdom
- MC_UU_00017/5/MRC_/Medical Research Council/United Kingdom
- SP/14/3/31114/BHF_/British Heart Foundation/United Kingdom
- SP/08/010/25939/BHF_/British Heart Foundation/United Kingdom
- PG/05/013/18296/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical