Trifluridine/tipiracil+bevacizumab (BEV) vs. fluoropyrimidine-irinotecan+BEV as second-line therapy for metastatic colorectal cancer: a randomised noninferiority trial
- PMID: 36871043
- PMCID: PMC10147634
- DOI: 10.1038/s41416-023-02212-2
Trifluridine/tipiracil+bevacizumab (BEV) vs. fluoropyrimidine-irinotecan+BEV as second-line therapy for metastatic colorectal cancer: a randomised noninferiority trial
Abstract
Background: This open-label, multicentre, phase II/III trial assessed the noninferiority of trifluridine/tipiracil (FTD/TPI) plus bevacizumab vs. fluoropyrimidine and irinotecan plus bevacizumab (control) as second-line treatment for metastatic colorectal cancer (mCRC).
Methods: Patients were randomised (1:1) to receive FTD/TPI (35 mg/m2 twice daily, days 1-5 and days 8-12, 28-day cycle) plus bevacizumab (5 mg/kg, days 1 and 15) or control. The primary endpoint was overall survival (OS). The noninferiority margin of the hazard ratio (HR) was set to 1.33.
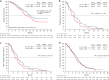
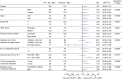
Results: Overall, 397 patients were enrolled. Baseline characteristics were similar between the groups. Median OS was 14.8 vs. 18.1 months (FTD/TPI plus bevacizumab vs. control; HR 1.38; 95% confidence interval [CI] 0.99-1.93; Pnoninferiority = 0.5920). In patients with a baseline sum of the diameter of target lesions of <60 mm (n = 216, post hoc analyses), the adjusted median OS was similar between groups (FTD/TPI plus bevacizumab vs. control, 21.4 vs. 20.7 months; HR 0.92; 95% CI 0.55-1.55). Grade ≥3 adverse events (FTD/TPI plus bevacizumab vs. control) included neutropenia (65.8% vs. 41.6%) and diarrhoea (1.5% vs. 7.1%).
Conclusions: FTD/TPI plus bevacizumab did not demonstrate noninferiority to fluoropyrimidine and irinotecan plus bevacizumab as second-line treatment for mCRC.
Clinical trial registration: JapicCTI-173618, jRCTs031180122.
© 2023. The Author(s).
Conflict of interest statement
YKu has received (in the past 36 months) institutional grants or contracts from Taiho, Takeda, Ono, AbbVie, AstraZeneca, Boehringer Ingelheim, Incyte, Amgen, Chugai, GlaxoSmithKline, Genmab, Astellas, and Daiichi Sankyo; personal consulting fees from Taiho, Takeda, and Boehringer Ingelheim; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Taiho, Ono, Bayer, and Sanofi. TT has received (in the past 36 months) payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Chugai, Eli Lilly Japan, Taiho Pharmaceutical, and Sanofi; has been part of the data safety monitoring board or advisory board of Sanofi; and is an employee for Shionogi. TM has received (in the past 36 months) institutional grants or contracts from MSD, Daiichi Sankyo, Ono, and Novartis and personal consulting fees from Takeda, Chugai, Merck Biopharma, Taiho, Bayer, Eli Lilly Japan, Yakult Honsha, Sanofi, Daiichi Sankyo, Ono, and Bristol Myers Squibb. MN has received (in the past 36 months) payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Bayer, Daiichi Sankyo, Merck Biopharma, Taiho Pharmaceutical, Yakult, Chugai, Eli Lilly Japan, Ono, and Takeda. JW has received (in the past 36 months) institutional grants or contracts from Medtronic, Amco, and Terumo and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Medtronic, Johnson & Johnson, and Eli Lilly. AM has received (in the past 36 months) payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Eli Lilly Japan, Ono, Daiichi Sankyo, Taiho, and Bristol Myers Squibb. MK has received (in the past 36 months) payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Chugai, Taiho, and Yakult. HH has received (in the past 36 months) institutional grants from Amgen, AstraZeneca, BeiGene, Chugai, Sumitomo Dainippon, Janssen, MSD, Taiho, Astellas, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Eisai, Merck Biopharma, and Ono; personal consulting fees from Bristol Myers Squibb, Daiichi Sankyo, MSD, Boehringer Ingelheim, Sumitomo Dainippon, and Ono; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Bayer, Chugai, Kyowa Kirin, Merck Biopharma, Ono, Taiho, Yakult, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, MSD, Sanofi, and Takeda. YKa has received (in the past 36 months) payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Taiho, Eli Lilly, Chugai, Takeda, Ono, Yakult, Sanofi, Merck, and MSD. HK has received (in the past 36 months) institutional grants or contracts from Chugai Pharmaceutical, Kobayashi Pharmaceutical Co. Ltd., Taiho, and Eisai; personal consulting fees from Daiichi Sankyo; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Bristol Myers Squibb, Eli Lilly Japan, Ono, Daiichi Sankyo, Takeda, Teijin Pharma Ltd., GlaxoSmithKline K.K., Bayer, MSD K.K., Chugai, Merck Biopharma, Yakult, Taiho, and Otsuka. AT has received (in the past 36 months) institutional grants or contracts from MSD, Daiichi Sankyo, Taiho Pharmaceutical, Hutchison MediPharma, Incyte, Ono, Sumitomo Dainippon, Pfizer Inc., and Isofol Medical AB and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Chugai Pharmaceutical, Taiho Pharmaceutical, Ono, Eli Lilly, and MSD. TK has received (in the past 36 months) payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Taiho Pharmaceutical, Chugai Pharmaceutical, Ono, and Eli Lilly Japan. EO has received (in the past 36 months) payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Taiho. YS has received (in the past 36 months) institutional grants or contracts from Chugai, Taiho, Takeda, Sanofi, Otsuka, and Eli Lilly Japan; payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Eli Lilly Japan, Bristol Myers Squibb, Chugai, Takeda, Ono, Merck Biopharma, Taiho, Bayer, Daiichi Sankyo, MSD, Sysmex, and Guardant Health; and has participated on the data safety monitoring board or advisory board of Daiichi Sankyo, MSD, and Guardant Health. SI has received (in the past 36 months) institutional grants or contracts from Yakult and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Taiho and Yakult. HT has received (in the past 36 months) institutional grants or contracts from Takeda, Daiichi Sankyo, and Ono and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Takeda, Taiho, Eli Lilly Japan, Merck Biopharma, Ono, and Chugai. TEN has received (in the past 36 months) institutional royalties or licenses from Sumitomo Dainippon, Ono, Taiho, Takeda, Chugai, Sanofi K.K., Nippon Kayaku Co., MSD K.K., and Eli Lilly Japan; personal consulting fees from Thyas Co. Ltd. and Rebirthel Co. Ltd.; and payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Sumitomo Dainippon, Boehringer Ingelheim, Bristol Myers Squibb, Ono, Taiho, Amgen, Takeda, Chugai, Sanofi K.K., Novartis Japan, Nippon Kayaku Co., MSD K.K., Eli Lilly Japan, Bayer, Pfizer Japan Inc., Daiichi Sankyo, Yakult, Nipro Co., Merck Serono Co., AstraZeneca, IQVIA, and GlaxoSmithKline. SM has received (in the past 36 months) payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from AstraZeneca K.K., Bristol Myers Squibb Company, Chugai, Eli Lilly Japan, MSD K.K., Nippon Boehringer Ingelheim, Novartis Pharma K.K., Ono, Pfizer Japan Inc., and Taiho. NT and DO are employees of Taiho. TY has received (in the past 36 months) institutional grants or contracts from Ono, Sanofi K.K., Daiichi Sankyo, Parexel International Inc., Pfizer Japan Inc., Taiho Pharmaceutical, MSD K.K., Amgen K.K., Genomedia Inc., Sysmex Corporation, Chugai, and Nippon Boehringer Ingelheim and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Taiho, Chugai, Eli Lilly Japan, Merck Biopharma, Bayer, Ono, and MSD K.K. HO, NS, and KS have nothing to disclose.
Figures


References
-
- Xu W, He Y, Wang Y, Li X, Young J, Ioannidis JPA, et al. Risk factors and risk prediction models for colorectal cancer metastasis and recurrence: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:172. doi: 10.1186/s12916-020-01618-6. - DOI - PMC - PubMed
-
- Shinozaki E, Makiyama A, Kagawa Y, Satake H, Tanizawa Y, Cai Z, et al. Treatment sequences of patients with advanced colorectal cancer and use of second-line FOLFIRI with antiangiogenic drugs in Japan: a retrospective observational study using an administrative database. PLoS One. 2021;16:e0246160. doi: 10.1371/journal.pone.0246160. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

