Striatal Subregion-selective Dysregulated Dopamine Receptor-mediated Intracellular Signaling in a Model of DOPA-responsive Dystonia
- PMID: 36871883
- PMCID: PMC10085842
- DOI: 10.1016/j.neuroscience.2023.02.020
Striatal Subregion-selective Dysregulated Dopamine Receptor-mediated Intracellular Signaling in a Model of DOPA-responsive Dystonia
Abstract
Although the mechanisms underlying dystonia are largely unknown, dystonia is often associated with abnormal dopamine neurotransmission. DOPA-responsive dystonia (DRD) is a prototype disorder for understanding dopamine dysfunction in dystonia because it is caused by mutations in genes necessary for the synthesis of dopamine and alleviated by the indirect-acting dopamine agonist l-DOPA. Although adaptations in striatal dopamine receptor-mediated intracellular signaling have been studied extensively in models of Parkinson's disease, another movement disorders associated with dopamine deficiency, little is known about dopaminergic adaptations in dystonia. To identify the dopamine receptor-mediated intracellular signaling associated with dystonia, we used immunohistochemistry to quantify striatal protein kinase A activity and extracellular signal-related kinase (ERK) phosphorylation after dopaminergic challenges in a knockin mouse model of DRD. l-DOPA treatment induced the phosphorylation of both protein kinase A substrates and ERK largely in D1 dopamine receptor-expressing striatal neurons. As expected, this response was blocked by pretreatment with the D1 dopamine receptor antagonist SCH23390. The D2 dopamine receptor antagonist raclopride also significantly reduced the phosphorylation of ERK; this contrasts with models of parkinsonism in which l-DOPA-induced ERK phosphorylation is not mediated by D2 dopamine receptors. Further, the dysregulated signaling was dependent on striatal subdomains whereby ERK phosphorylation was largely confined to dorsomedial (associative) striatum while the dorsolateral (sensorimotor) striatum was unresponsive. This complex interaction between striatal functional domains and dysregulated dopamine-receptor mediated responses has not been observed in other models of dopamine deficiency, such as parkinsonism, suggesting that regional variation in dopamine-mediated neurotransmission may be a hallmark of dystonia.
Keywords: dopamine; dystonia; extracellular signal-related kinase; protein kinase A; striatum.
Copyright © 2023 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors declare no conflict of interests.
Figures


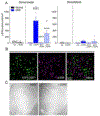
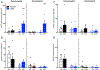

References
-
- Alcacer C, Santini E, Valjent E, Gaven F, Girault JA, & Herve D (2012). Galpha(olf) mutation allows parsing the role of cAMP-dependent and extracellular signal-regulated kinase-dependent signaling in L-3,4-dihydroxyphenylalanine-induced dyskinesia. J Neurosci, 32(17), 5900–5910. 10.1523/JNEUROSCI.0837-12.2012 - DOI - PMC - PubMed
-
- Braz BY, Galinanes GL, Taravini IR, Belforte JE, & Murer MG (2015). Altered Corticostriatal Connectivity and Exploration/Exploitation Imbalance Emerge as Intermediate Phenotypes for a Neonatal Dopamine Dysfunction. Neuropsychopharmacology, 40(11), 2576–2587. 10.1038/npp.2015.104 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

