Depilatory double-disc mouse model for evaluation of vesicant dermal injury pharmacotherapy countermeasures
- PMID: 36872306
- PMCID: PMC9986227
- DOI: 10.1002/ame2.12304
Depilatory double-disc mouse model for evaluation of vesicant dermal injury pharmacotherapy countermeasures
Abstract
Background: Sulfur mustard (SM) is a chemical warfare vesicant that severely injures exposed eyes, lungs, and skin. Mechlorethamine hydrochloride (NM) is widely used as an SM surrogate. This study aimed to develop a depilatory double-disc (DDD) NM skin burn model for investigating vesicant pharmacotherapy countermeasures.
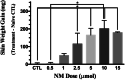
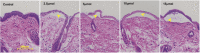
Methods: Hair removal method (clipping only versus clipping followed by a depilatory), the effect of acetone in the vesicant administration vehicle, NM dose (0.5-20 μmol), vehicle volume (5-20 μl), and time course (0.5-21 days) were investigated using male and female CD-1 mice. Edema, an indicator of burn response, was assessed by biopsy skin weight. The ideal NM dose to induce partial-thickness burns was assessed by edema and histopathologic evaluation. The optimized DDD model was validated using an established reagent, NDH-4338, a cyclooxygenase, inducible nitric oxide synthase, and acetylcholinesterase inhibitor prodrug.
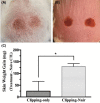
Results: Clipping/depilatory resulted in a 5-fold higher skin edematous response and was highly reproducible (18-fold lower %CV) compared to clipping alone. Acetone did not affect edema formation. Peak edema occurred 24-48 h after NM administration using optimized dosing methods and volume. Ideal partial-thickness burns were achieved with 5 μmol of NM and responded to treatment with NDH-4338. No differences in burn edematous responses were observed between males and females.
Conclusion: A highly reproducible and sensitive partial-thickness skin burn model was developed for assessing vesicant pharmacotherapy countermeasures. This model provides clinically relevant wound severity and eliminates the need for organic solvents that induce changes to the skin barrier function.
Keywords: edema; mice; skin; vesicant.
© 2023 The Authors. Animal Models and Experimental Medicine published by John Wiley & Sons Australia, Ltd on behalf of The Chinese Association for Laboratory Animal Sciences.
Conflict of interest statement
The authors declare there are no conflicts of interest.
Figures



Similar articles
-
Mitigation of nitrogen mustard mediated skin injury by a novel indomethacin bifunctional prodrug.Exp Mol Pathol. 2016 Jun;100(3):522-31. doi: 10.1016/j.yexmp.2016.05.008. Epub 2016 May 14. Exp Mol Pathol. 2016. PMID: 27189522 Free PMC article.
-
Histopathological and immunohistochemical evaluation of nitrogen mustard-induced cutaneous effects in SKH-1 hairless and C57BL/6 mice.Exp Toxicol Pathol. 2014 Mar;66(2-3):129-38. doi: 10.1016/j.etp.2013.11.005. Epub 2013 Dec 25. Exp Toxicol Pathol. 2014. PMID: 24373750 Free PMC article.
-
Mustard vesicants alter expression of the endocannabinoid system in mouse skin.Toxicol Appl Pharmacol. 2016 Jul 15;303:30-44. doi: 10.1016/j.taap.2016.04.014. Epub 2016 Apr 26. Toxicol Appl Pharmacol. 2016. PMID: 27125198 Free PMC article.
-
Research models of sulfur mustard- and nitrogen mustard-induced ocular injuries and potential therapeutics.Exp Eye Res. 2022 Oct;223:109209. doi: 10.1016/j.exer.2022.109209. Epub 2022 Aug 9. Exp Eye Res. 2022. PMID: 35961426 Review.
-
Mustard vesicating agent-induced toxicity in the skin tissue and silibinin as a potential countermeasure.Ann N Y Acad Sci. 2016 Jun;1374(1):184-92. doi: 10.1111/nyas.13099. Epub 2016 Jun 21. Ann N Y Acad Sci. 2016. PMID: 27326543 Free PMC article. Review.
Cited by
-
Optimizing Nanosuspension Drug Release and Wound Healing Using a Design of Experiments Approach: Improving the Drug Delivery Potential of NDH-4338 for Treating Chemical Burns.Pharmaceutics. 2024 Mar 27;16(4):471. doi: 10.3390/pharmaceutics16040471. Pharmaceutics. 2024. PMID: 38675132 Free PMC article.
References
-
- Ghabili K, Agutter PS, Ghanei M, Ansarin K, Panahi Y, Shoja MM. Sulfur mustard toxicity: history, chemistry, pharmacokinetics, and pharmacodynamics. Crit Rev Toxicol. 2011;41(5):384‐403. - PubMed
-
- Mangerich A, Debiak M, Birtel M, et al. Sulfur and nitrogen mustards induce characteristic poly(ADP‐ribosyl)ation responses in HaCaT keratinocytes with distinctive cellular consequences. Toxicol Lett. 2016;244:56‐71. - PubMed
-
- Weber GF. DNA damaging drugs. In: Molecular Therapies of Cancer. Springer; 2015:9‐112.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

