Pembrolizumab near the end of life in patients with metastatic pancreatic cancer: a multi-site consecutive series to examine survival and patient treatment burden
- PMID: 36872382
- PMCID: PMC10272060
- DOI: 10.1007/s00262-023-03397-4
Pembrolizumab near the end of life in patients with metastatic pancreatic cancer: a multi-site consecutive series to examine survival and patient treatment burden
Abstract
Background: Pembrolizumab confers minimal benefit to most patients with pancreas cancer. We explored survival and patient treatment burden (for example, death within 14 days of therapy) in a subgroup who had early access to pembrolizumab .
Methods: This multisite study examined consecutive pancreas cancer patients, who received pembrolizumab from 2004 through 2022. Median overall survival of > 4 months was to be deemed favorable. Patient treatment burden and medical record quotations are presented descriptively.
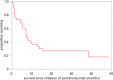
Results: Forty-one patients (median age 66 years; range 36, 84) are included. Fifteen (37%) had dMMR, MSI-H, TMB-H, or Lynch syndrome; and 23 (56%) received concurrent therapy. The median overall survival was 7.2 months (95% confidence interval (CI): 5.2, 12.7 months); 29 were deceased at the time of reporting. Patients with dMMR, MSI-H, TMB-H, or Lynch syndrome had a lower risk of death: hazard ratio (HR): 0.29 (95% CI: 0.12, 0.72); p = 0.008. Medical record phrases ("brilliant response") aligned with the above. One patient died within 14 days of therapy, and one was in an intensive care unit within 30 days of death. Fifteen patients enrolled in hospice; four of these died < 3 days later.
Conclusions: These unexpectedly favorable findings underscore the need for healthcare providers-including palliative care providers-to knowledgeably guide patients about cancer therapy even near the end of life.
Keywords: End of life; Metastatic; Palliation; Pancreas cancer; Pembrolizumab.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

