Acute deletion of the central MR/GR steroid receptor correlates with changes in LTP, auditory neural gain, and GC-A cGMP signaling
- PMID: 36873102
- PMCID: PMC9983609
- DOI: 10.3389/fnmol.2023.1017761
Acute deletion of the central MR/GR steroid receptor correlates with changes in LTP, auditory neural gain, and GC-A cGMP signaling
Abstract
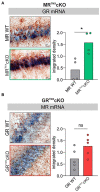
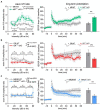
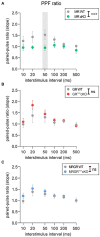
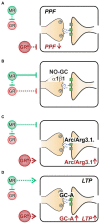
The complex mechanism by which stress can affect sensory processes such as hearing is still poorly understood. In a previous study, the mineralocorticoid (MR) and/or glucocorticoid receptor (GR) were deleted in frontal brain regions but not cochlear regions using a CaMKIIα-based tamoxifen-inducible Cre ERT2/loxP approach. These mice exhibit either a diminished (MRTMXcKO) or disinhibited (GRTMXcKO) auditory nerve activity. In the present study, we observed that mice differentially were (MRTMXcKO) or were not (GRTMXcKO) able to compensate for altered auditory nerve activity in the central auditory pathway. As previous findings demonstrated a link between central auditory compensation and memory-dependent adaptation processes, we analyzed hippocampal paired-pulse facilitation (PPF) and long-term potentiation (LTP). To determine which molecular mechanisms may impact differences in synaptic plasticity, we analyzed Arc/Arg3.1, known to control AMPA receptor trafficking, as well as regulators of tissue perfusion and energy consumption (NO-GC and GC-A). We observed that the changes in PPF of MRTMXcKOs mirrored the changes in their auditory nerve activity, whereas changes in the LTP of MRTMXcKOs and GRTMXcKOs mirrored instead the changes in their central compensation capacity. Enhanced GR expression levels in MRTMXcKOs suggest that MRs typically suppress GR expression. We observed that hippocampal LTP, GC-A mRNA expression levels, and ABR wave IV/I ratio were all enhanced in animals with elevated GR (MRTMXcKOs) but were all lower or not mobilized in animals with impaired GR expression levels (GRTMXcKOs and MRGRTMXcKOs). This suggests that GC-A may link LTP and auditory neural gain through GR-dependent processes. In addition, enhanced NO-GC expression levels in MR, GR, and MRGRTMXcKOs suggest that both receptors suppress NO-GC; on the other hand, elevated Arc/Arg3.1 levels in MRTMXcKOs and MRGRTMXcKOs but not GRTMXcKOs suggest that MR suppresses Arc/Arg3.1 expression levels. Conclusively, MR through GR inhibition may define the threshold for hemodynamic responses for LTP and auditory neural gain associated with GC-A.
Keywords: GC-A; NO-GC; cognition; glucocorticoid receptor; mineralocorticoid receptor.
Copyright © 2023 Calis, Hess, Marchetta, Singer, Modro, Nelissen, Prickaerts, Sandner, Lukowski, Ruth, Knipper and Rüttiger.
Conflict of interest statement
PS was employed by the company BAYER. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Loss of central mineralocorticoid or glucocorticoid receptors impacts auditory nerve processing in the cochlea.iScience. 2022 Feb 26;25(3):103981. doi: 10.1016/j.isci.2022.103981. eCollection 2022 Mar 18. iScience. 2022. PMID: 35281733 Free PMC article.
-
Conditional Ablation of Glucocorticoid and Mineralocorticoid Receptors from Cochlear Supporting Cells Reveals Their Differential Roles for Hearing Sensitivity and Dynamics of Recovery from Noise-Induced Hearing Loss.Int J Mol Sci. 2023 Feb 7;24(4):3320. doi: 10.3390/ijms24043320. Int J Mol Sci. 2023. PMID: 36834731 Free PMC article.
-
Stress Affects Central Compensation of Neural Responses to Cochlear Synaptopathy in a cGMP-Dependent Way.Front Neurosci. 2022 Jul 29;16:864706. doi: 10.3389/fnins.2022.864706. eCollection 2022. Front Neurosci. 2022. PMID: 35968392 Free PMC article.
-
Brain corticosteroid receptor balance in health and disease.Endocr Rev. 1998 Jun;19(3):269-301. doi: 10.1210/edrv.19.3.0331. Endocr Rev. 1998. PMID: 9626555 Review.
-
Roles of the Glucocorticoid and Mineralocorticoid Receptors in Skin Pathophysiology.Int J Mol Sci. 2018 Jun 29;19(7):1906. doi: 10.3390/ijms19071906. Int J Mol Sci. 2018. PMID: 29966221 Free PMC article. Review.
Cited by
-
Repurposing Ketamine in the Therapy of Depression and Depression-Related Disorders: Recent Advances and Future Potential.Aging Dis. 2024 Apr 29;16(2):804-840. doi: 10.14336/AD.2024.0239. Aging Dis. 2024. PMID: 38916735 Free PMC article. Review.
-
Glucocorticoid receptor signaling in the brain and its involvement in cognitive function.Neural Regen Res. 2025 Sep 1;20(9):2520-2537. doi: 10.4103/NRR.NRR-D-24-00355. Epub 2024 Sep 6. Neural Regen Res. 2025. PMID: 39248182 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

